
ARROCase: Ewing Sarcoma
Jana Kobeissi, MS4
American University of Beirut, Lebanon
Mel Lizaso, MD
University of Cincinnati Cancer Center, Cincinnati, Ohio
Reviewed by: Luke Pater, MD
University of Cincinnati Cancer Center, Cincinnati, Ohio

Case Presentation
• 13 year old boy
• Localized, waxing & waning right leg pain
• 3 weeks duration
• Gradual onset
• Increasing in intensity
• ROS
• Otherwise negative
• No fever, night sweats, fatigue, weight loss

Case Presentation
• History unrevealing
• No history of trauma
• Negative past medical/surgical history
• Family history non-contributory
• No medications or known allergies
• Physical examination
• Afebrile, vital signs within normal range
• Point tenderness on palpation of mid-lateral side of right
leg with mild overlying swelling
• Normal gait and range of motion; Neuro exam non-focal
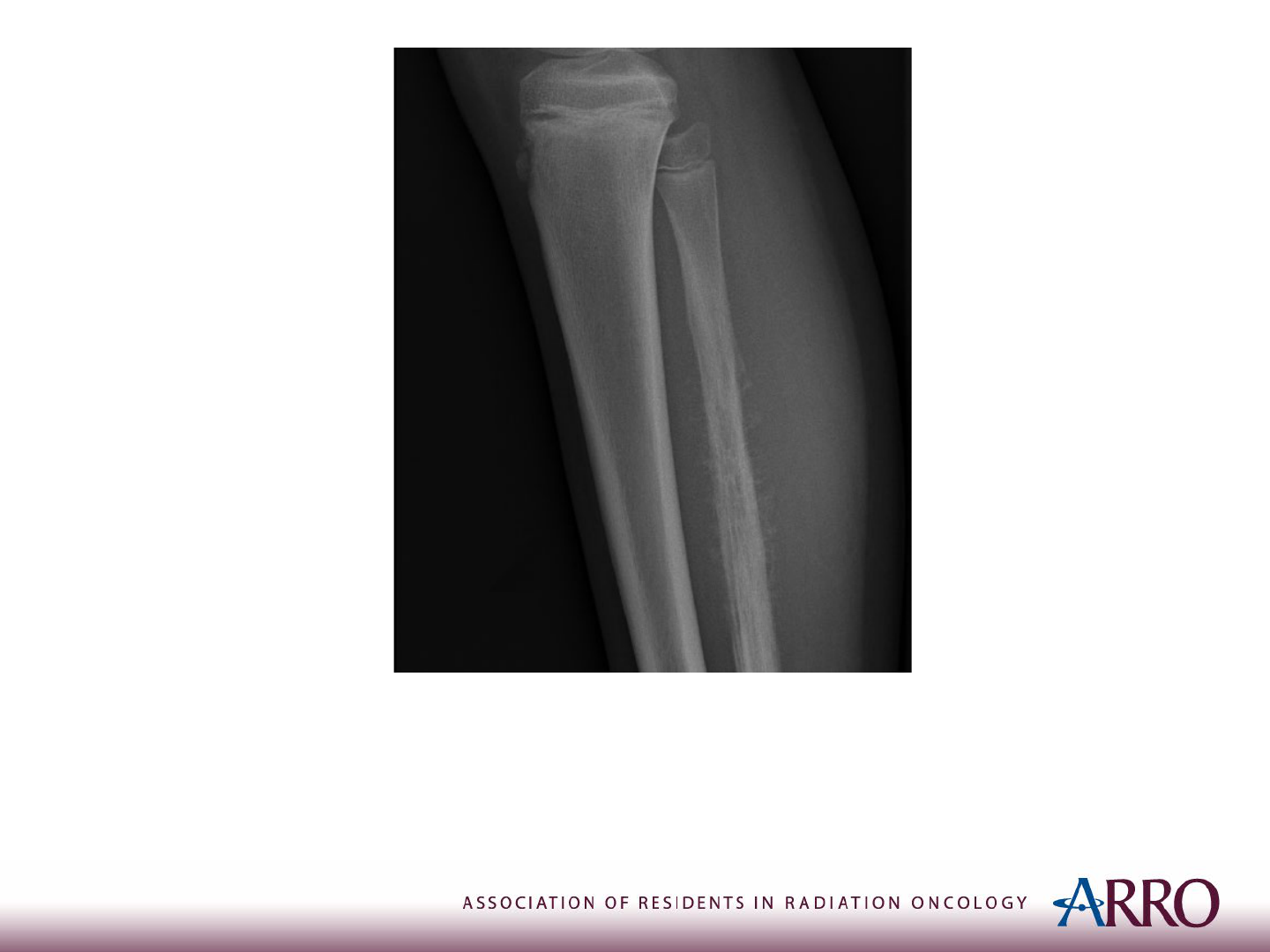
Imaging
X-ray of the Right Leg
Case courtesy of Dr Samir Benoudina, Radiopaedia.org, rID: 75437
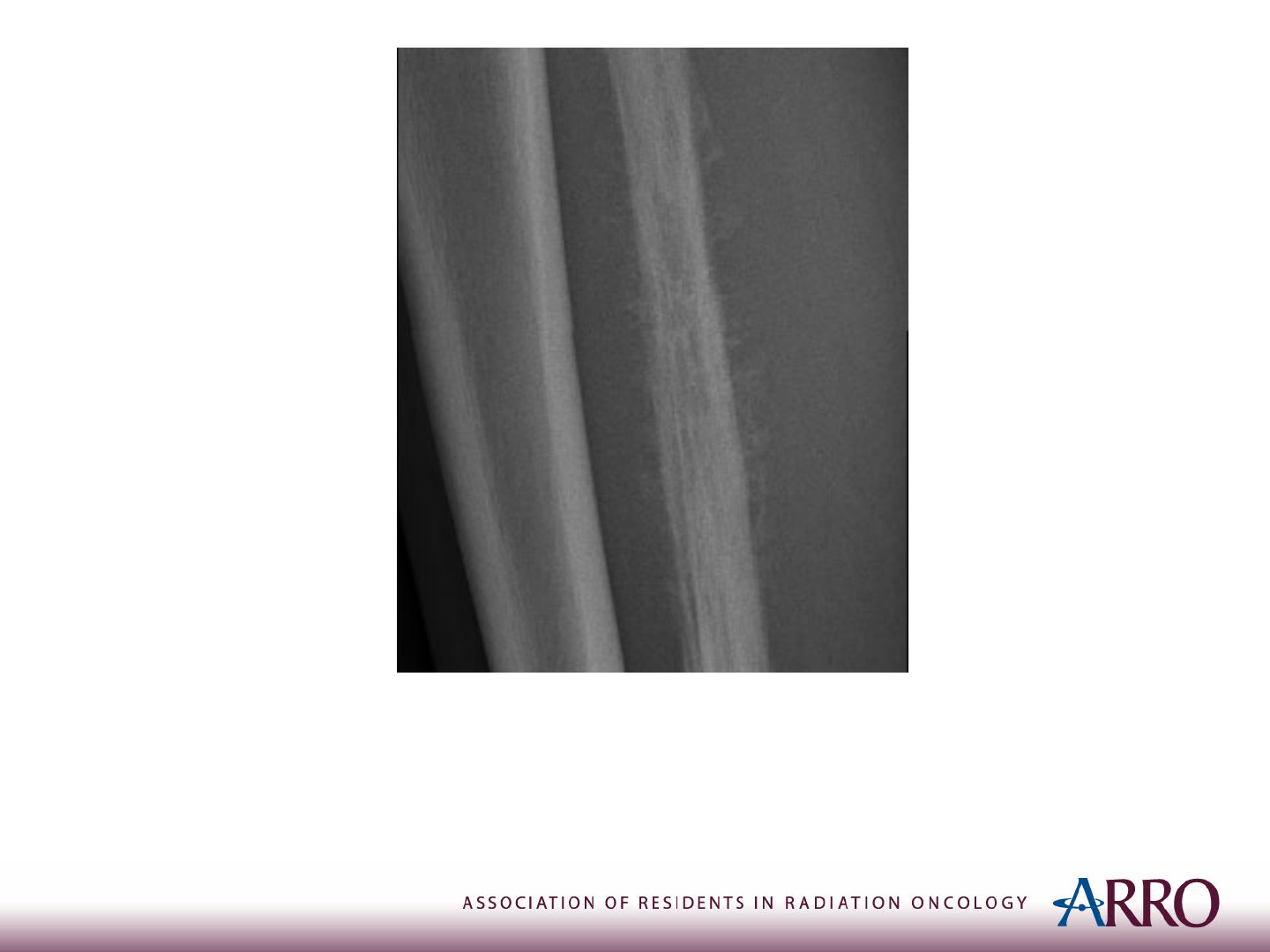
Imaging
X-ray of the Right Leg
Case courtesy of Dr Samir Benoudina, Radiopaedia.org, rID: 75437
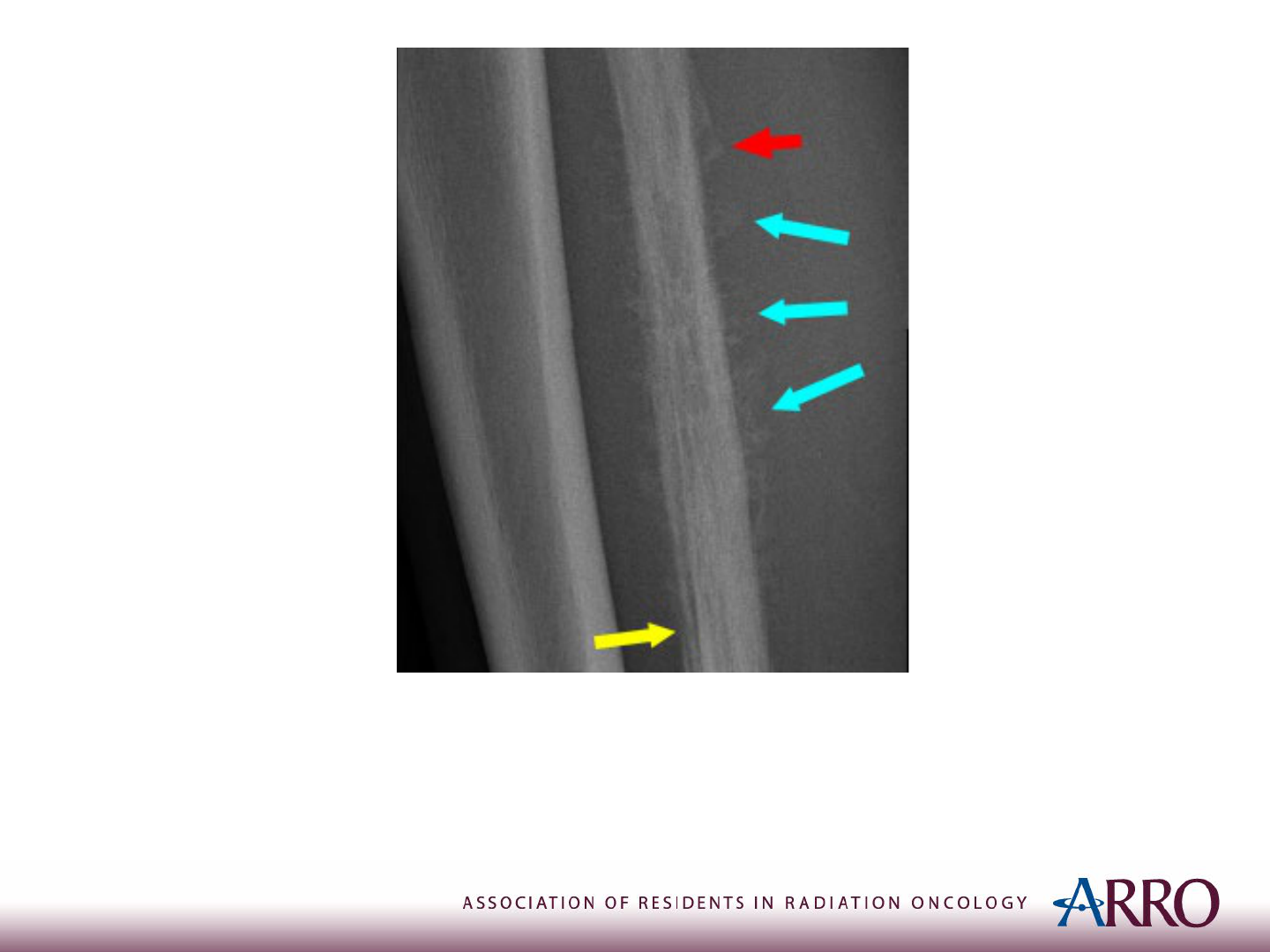
Imaging
X-ray of the Right Leg: Codman triangle (Red Arrow)
Sunburst Appearance (Blue Arrow)
Onion-Skin Periosteal reaction (Yellow Arrow)
Case courtesy of Dr Samir Benoudina, Radiopaedia.org, rID: 75437

Biopsy*
Sheets of small, uniform cells with scant cytoplasm, round nuclei, and
small punctate nucleoli
*Open incisional biopsy is preferred so that the biopsy tract can be
removed with definitive surgery.
https://www.webpathology.com/image.asp?n=5&Case=340

EWING SARCOMA
Diagnosis and Workup

Epidemiology
• Aggressive bone and soft-tissue cancer
• Predominant in children and adolescents
• Peak incidence at 15 years of age
• About 2% of all cancers in children
• Around 200 new cases/year
• Second most common bone cancer
• Male > Female
• About 25% present with metastatic disease

Presentation
• Localized pain and swelling
• Most commonly in pelvis and proximal
long bones
• Possible palpation of a firm mass
• Pathological fracture in 10-15% of cases
• Constitutional symptoms
• Fever, night sweats, fatigue, and weight
loss
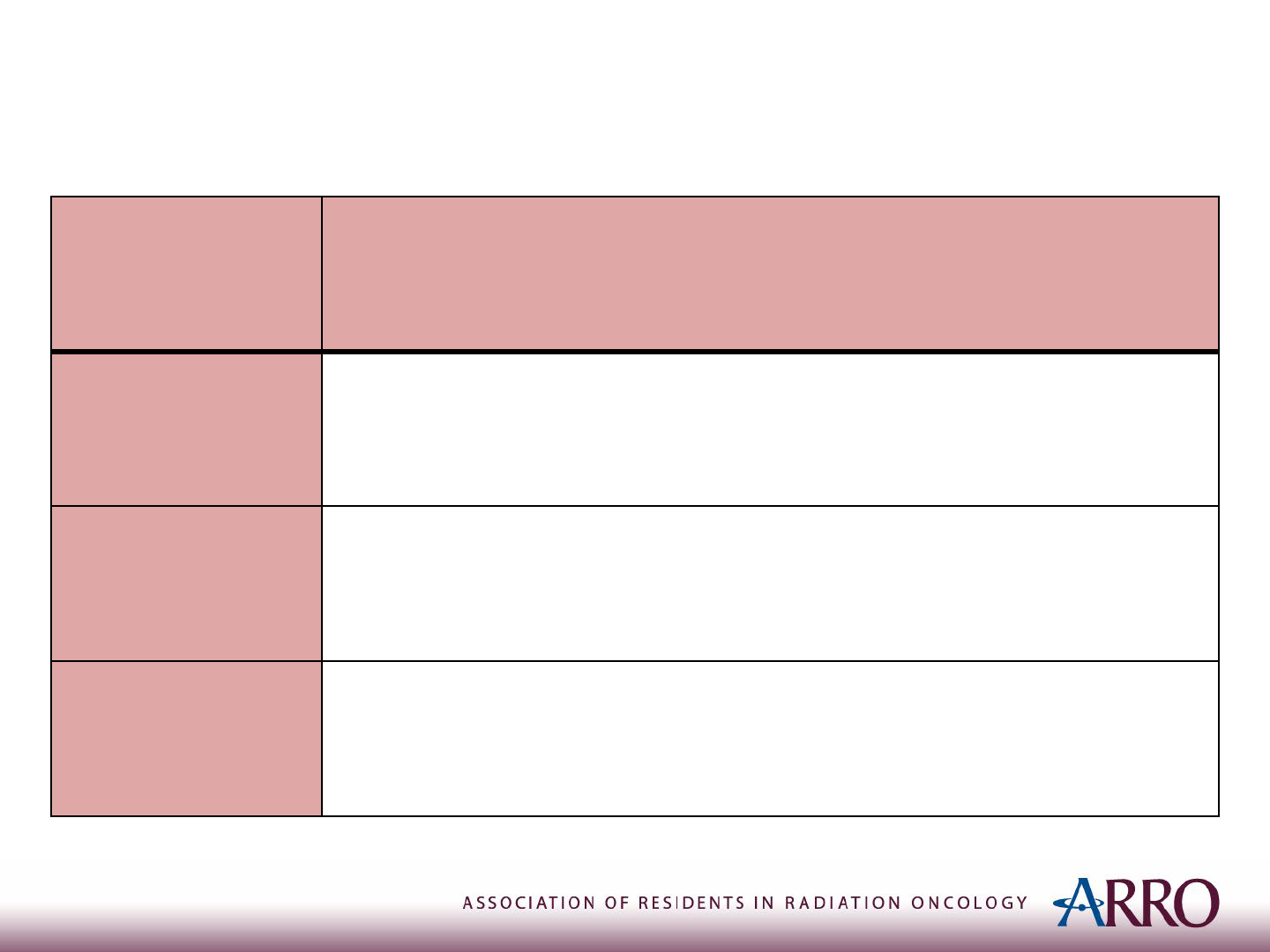
Diagnosis
TEST RESULT
Plain
radiograph
• Multiple confluent lytic lesions, like “Moth eaten” bone
• Periosteal reaction, giving rise to “onion peel” or Codman’s
triangle
Biopsy
• Sheets of small, round, blue cells with a prominent nucleus and
scant cytoplasm
Blood tests
• May show elevated levels of nonspecific markers of inflammation
and bone remodeling (ESR, Alk Phos)
• Elevated serum LDH

Differential Diagnosis for Small Round Blue
Cell Tumors
• Histologic findings shared with:
• Neuroblastoma
• Desmoplastic small round-cell tumor
• Alveolar rhabdomyosarcoma
• Peripheral neuroectodermal tumor
• Non-Hodgkin’s lymphoma
• Acute lymphoblastic leukemia
• Poorly differentiated synovial sarcoma
• Other rare “Ewing-like” tumors

Molecular Studies
• Fusion of:
• EWS gene (chr. 22)
• Gene of the ETS family
• Most common (85%):
• EWS & FLI-1 fusion; t(11;22)(q24;q12)
• Associated with a better prognosis
• Identification of signature chromosomal
translocation —> Definitive diagnosis
• Consider fusion panels if initial studies -ve

Prognostic Factors
• Favorable
• Distal lesion
• Tumor volume < 100 mL
• Normal LDH level
• Adverse
• Metastatic disease at presentation
• Poor response to initial chemotherapy

Staging
• MRI with or without CT of 1
o
lesion (with
contrast)
• Head-to-toe PET CT scan and/or bone scan
• In case of high risk disease or concerning
symptoms, consider:
• CT Chest with contrast
• Bone marrow biopsy
• Scanning MRI of spine and pelvis

Staging System
• Localized v.s. Metastatic
• Metastases can be
• Regional (nearby structures/lymph nodes)
• Distant (distant organs; eg, lung)
• TNM staging
• Less commonly used
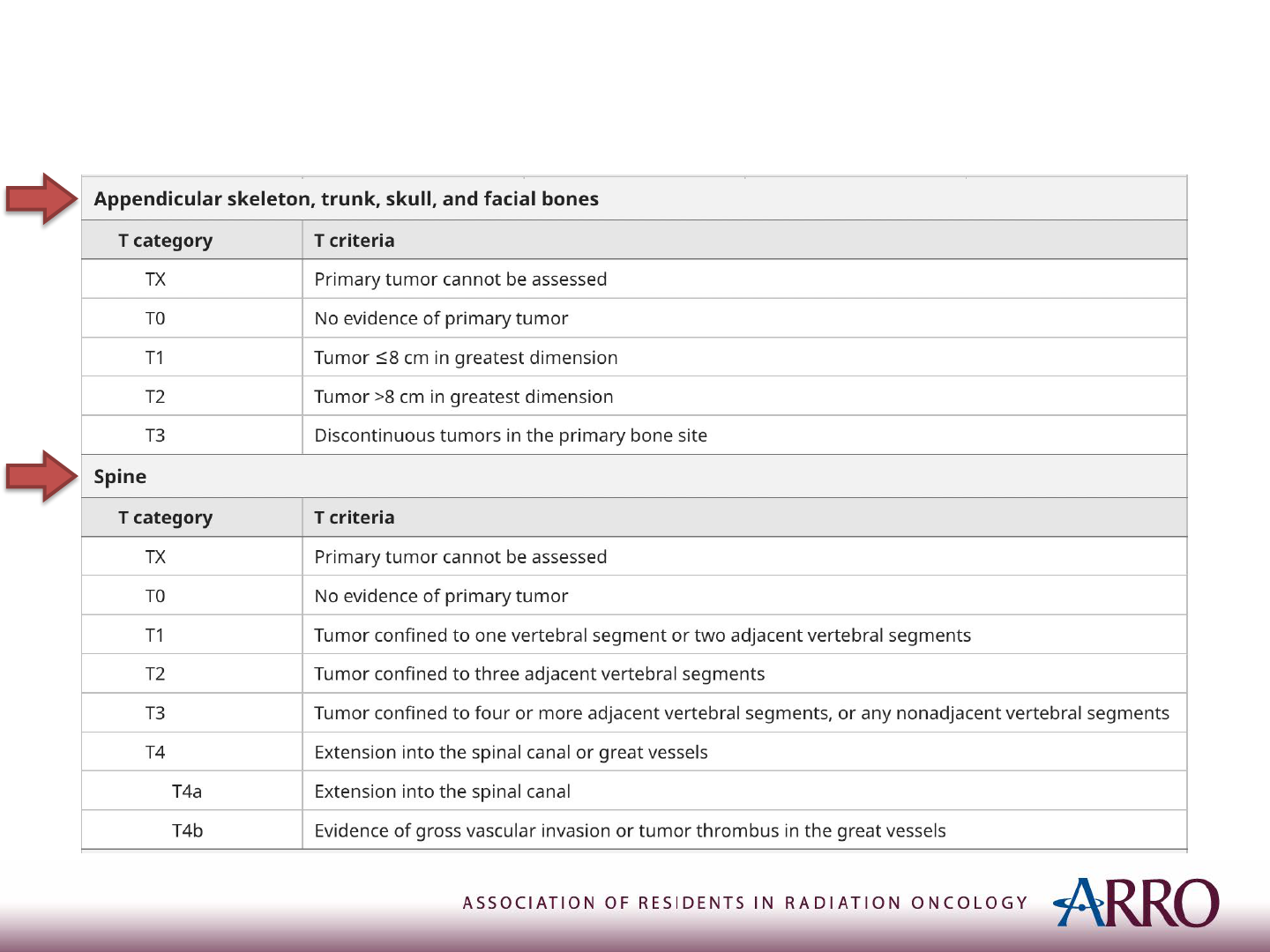
TNM Staging
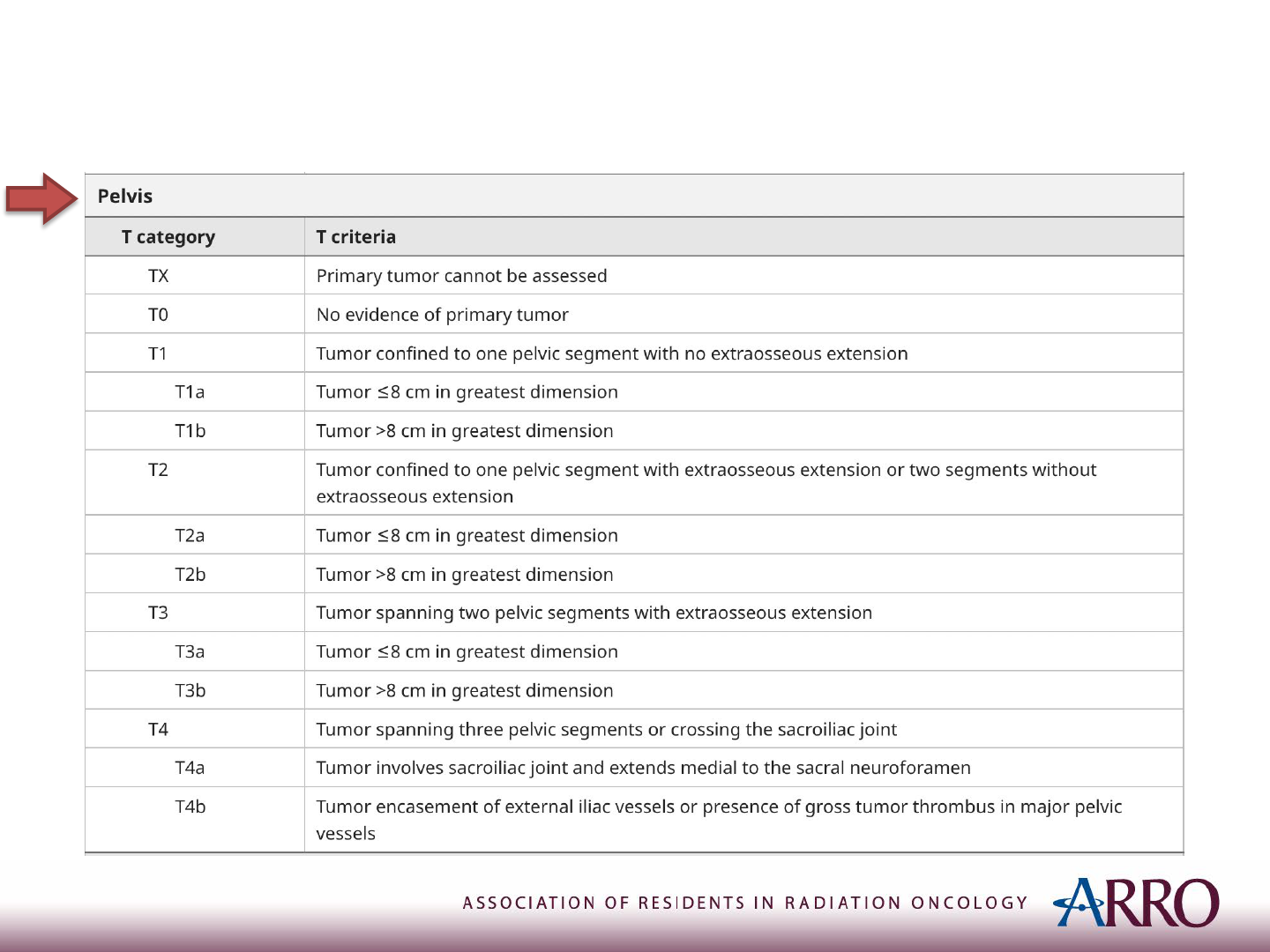
TNM Staging
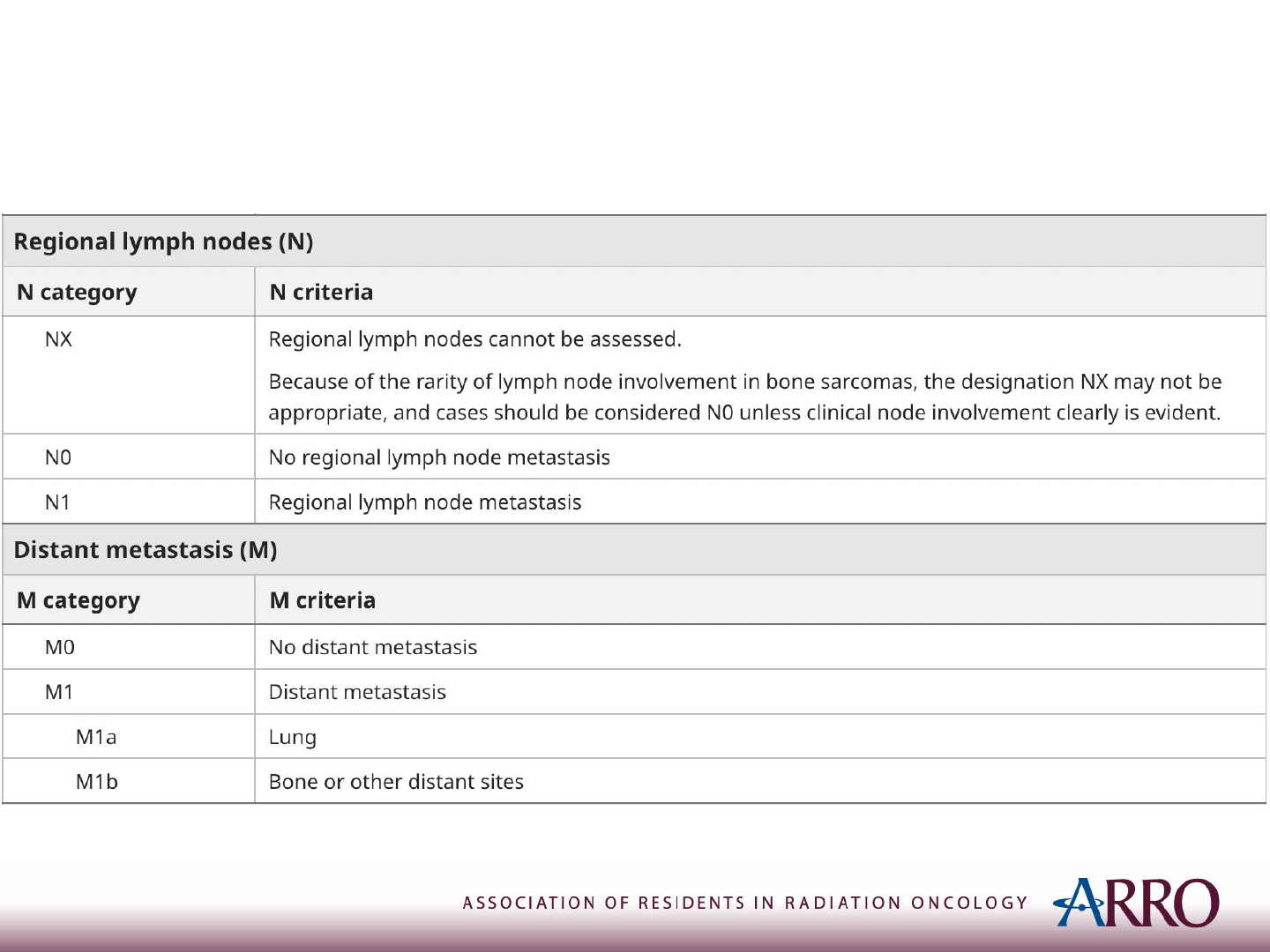
TNM Staging

Outcomes
• 5-year relative survival rates
• Localized: 82%
• Regional: 70%
• Distant: 39%
• All stages combined: 62%
American Cancer Society statistics: 2010-2016.

Outcomes
• Cure rate in case of metastases in:
• Lung: 30%
• Bone/bone marrow: 20%
• Local control rates:
• Surgery for extremity tumor: >90%
• Surgery for central tumor: 75%
• Radiation therapy: 75-90%

GUIDELINES ON MANAGEMENT
NCCN v2.2022

LOCALIZED EWING SARCOMA
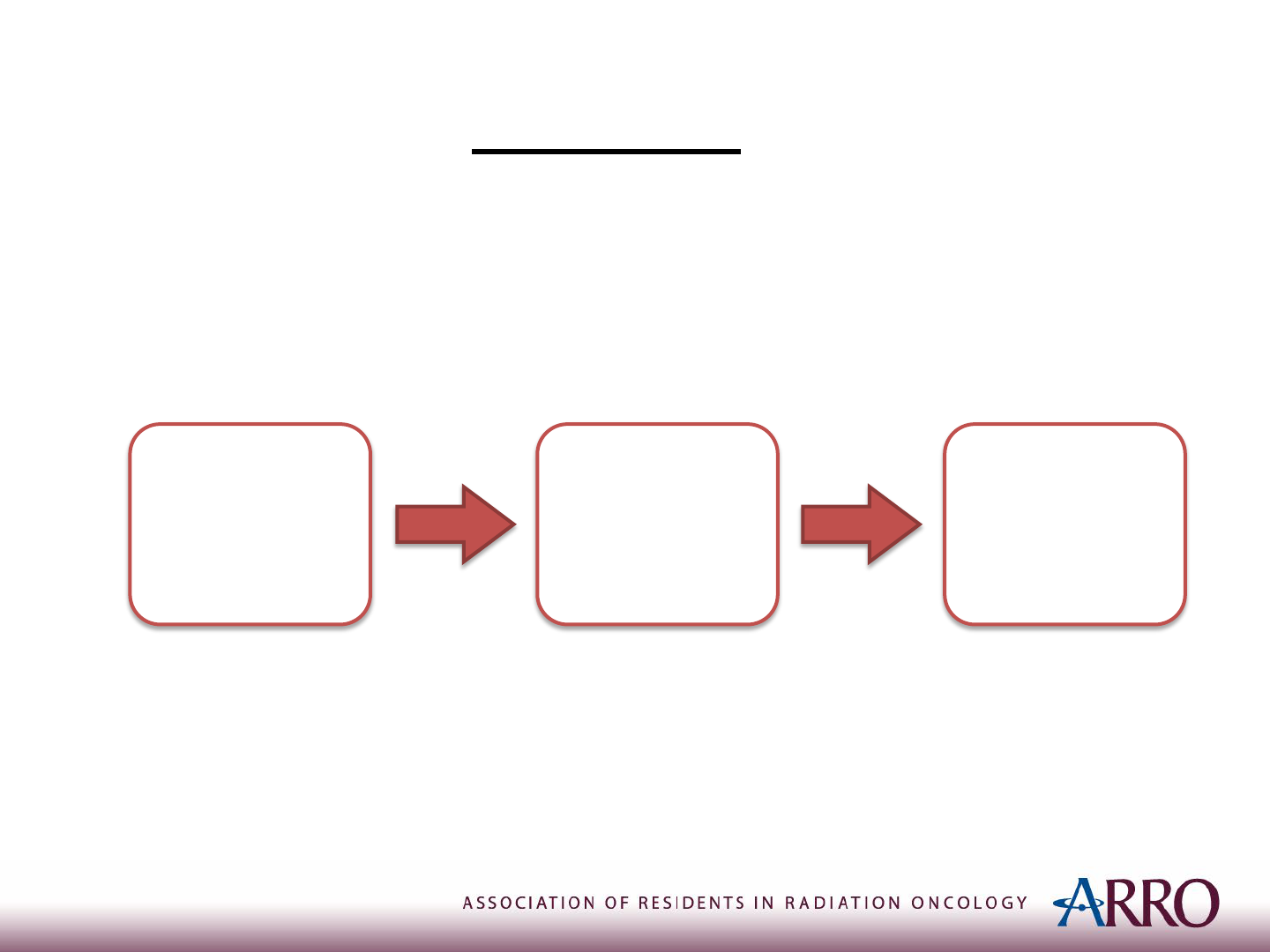
Treatment of Localized Ewing Sarcoma
• In brief:
Induction
Chemotherapy
Local
Treatment
Consolidation
Chemotherapy

Treatment of Localized Ewing Sarcoma
• Induction Chemotherapy
• Alternating VDC & IE
• Vincristine, Doxorubicin, Cyclophosphamide
• Ifosfamide, Etoposide
• Six Cycles
• Re-staging
• Repeat imaging of initial workup
• Stable/improved v.s. progressive
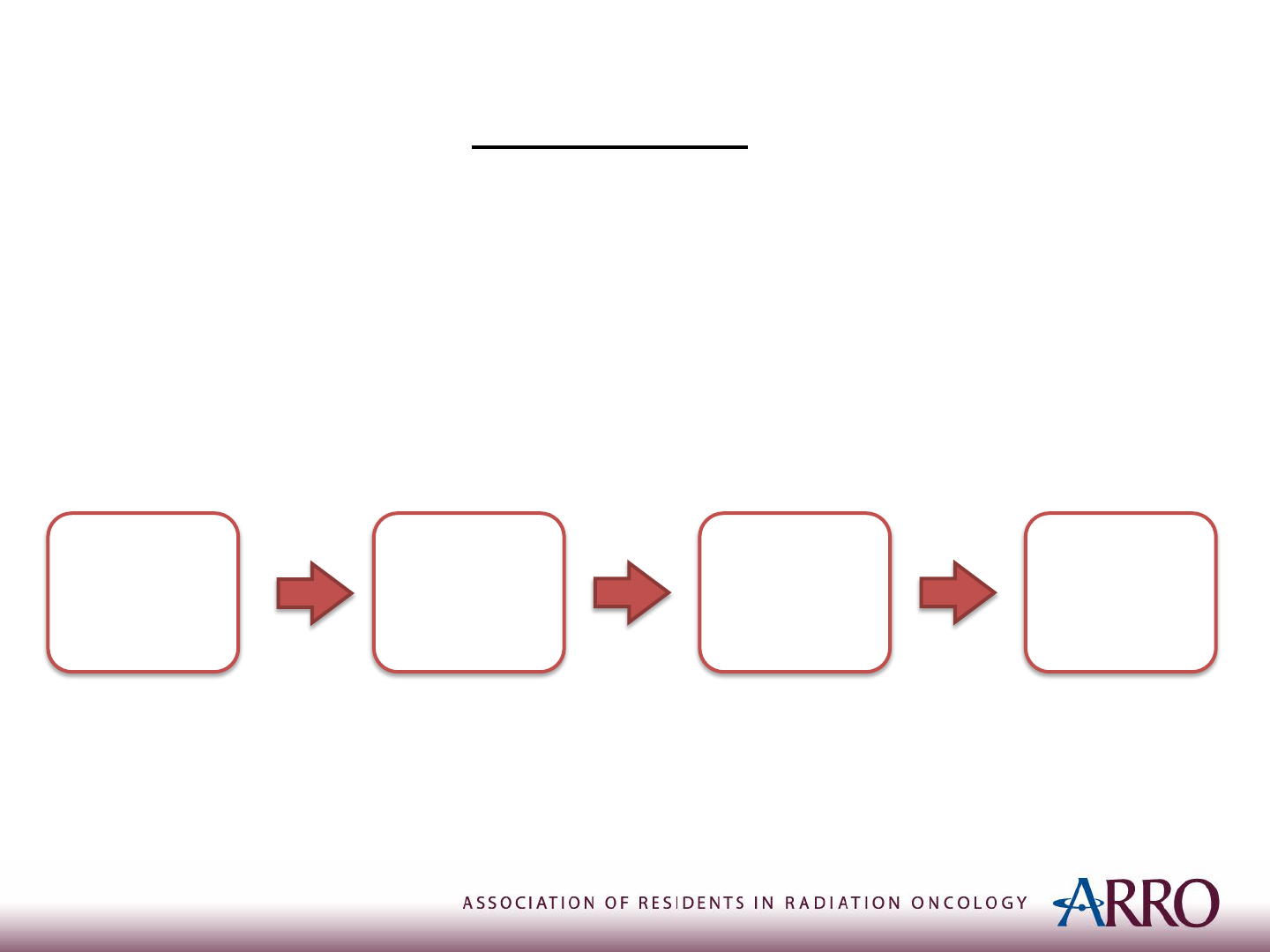
Treatment of Localized Ewing Sarcoma
• If stable or improved —> Local therapy at ~ 15
wks
• Surgery
• Wide local excision or amputation
• Followed by
• Chemo (regardless of surgical margins)
• + RT (if close or positive margins,
consider for pelvic tumors)
• OR Definitive chemoradiation

Treatment of Localized Ewing Sarcoma
• If progressive —> Consider local therapy
• RT or
• Surgery
• For local control or palliation

Treatment of Localized Ewing Sarcoma
• More on local therapy
• Prefer surgery for:
• Younger children (to avoid 2
nd
malignancy)
• Tumors in proximal fibula, lateral clavicle, ribs,
scapular body, iliac wings, small bones of the
hands/feet (i.e. “expendable” bones)
• Prefer definitive chemoRT for:
• Prevention of limb amputation
• Tumors in pelvis/spine (surgery would be debilitating)

Treatment of Localized Ewing Sarcoma
• Consolidation chemotherapy
• To be given after local therapy
• Alternating VDC & IE
• For 11 cycles

METASTATIC EWING SARCOMA

Treatment of Metastatic Ewing Sarcoma
• Primary Treatment: Chemotherapy
• Then:
• Consider local therapy, especially if:
• Oligometastatic
• Good response to chemotherapy
• Otherwise, if widely metastatic, consider:
• Chemo with palliative surgery
• Palliative RT to symptomatic areas
Treatment of Metastatic Ewing Sarcoma
• Special case:
Ewing sarcoma metastatic to lung only
• Treat definitively:
Induction
Chemotherapy
Local
Treatment
Consolidation
Chemotherapy
Consolidation
whole lung RT

RADIATION THERAPY
More on

Radiation Therapy
• Definitive RT
• GTV1: pretreatment bone + soft tissue (45 Gy at 1.8 Gy/fx)
• CTV1: 1-1.5 cm
• PTV1: 0.5-1 cm
• GTV2: post-chemotherapy soft tissue (55.8 Gy at 1.8 Gy/fx)
• CTV2: 1-1.5 cm
• PTV2: 0.5-1 cm
• N.B.
• Anatomically modified CTV so as not to cross nearby borders
• No need to expand into structures which the tumor abutted (but did not invade)
• PTV2 of vertebral body tumors receives 50.4 Gy instead
NCCN v2.2022
Donaldson. Pediatr Blood Cancer. 2004.
Radiation Therapy
• Preoperative RT
• For marginally resectable tumors to improve margin
status
• Eg, R1 —> potential R0
• Goal is not to downstage an un-resectable
tumor (Eg, R2 —> potential R1/R0)
• Initial GTV + 2 cm
• Dose: 36-45 Gy (1.8 Gy/fx)
NCCN v2.2022

Radiation Therapy
• Postoperative RT (Within 60 days) all at (1.8 Gy/fx)
• GTV2 (45 Gy) + CTV1 + PTV1
• R0 resection (No microscopic residual)
• Esp. if unfavorable histology
• R1 resection (Microscopic residual)
• R2 resection (Gross residual)
• With cone down to residual
• Total dose: 55.8 Gy to GTV2 + CTV2 + PTV2
• LN +ve areas
• Resected: 50.4 Gy
• Un-resected: 55.8 Gy
NCCN v2.2022
Donaldson. Pediatr Blood Cancer. 2004.

Radiation Therapy
• Hemithorax Irradiation
• Indication: Chest wall 1
o
tumor w/ extensive pleural involvement
• Dose:
• 15-20 Gy (1.5 Gy/fx) to ipsilateral whole lung and pleura; THEN
• 21.6 Gy (1.8 Gy/fx) to PTV1; AND
• 14.4 Gy boost to PTV2
• Note: same PTV1 & PTV2 as definitive RT expansions
• Whole Lung Irradiation
• Indication: pulmonary metastases after chemotherapy (even if
complete response) or surgical resection
• Dose: 15 Gy if <14 y.o. or 18 Gy if >14 y.o. (1.5 Gy/fx)
NCCN v2.2022

Radiation Therapy - Some Constraints
• Keep V40 < 66%
• To avoid pathological fracture
• Avoid circumferential RT & add skin strip
• To avoid lymphedema
• Keep in mind:
• Epiphyseal closure at ~ 20 Gy
• Ovarian failure at ~ 8 Gy
• —> Lead shielding or ovarian transposition out of field
• Testicular failure at ~ 2 Gy
• —> Lead shielding

Radiation Therapy - Side Effects
• Secondary malignancy
• Growth abnormalities
• Fibrosis/edema
• Hypoplasia of muscles
• Femoral head necrosis
• Pathologic fractures
• Infertility

Chemotherapy - Side Effects
• Regimen: alternating VDC & IE
• Secondary AML (DC & IE)
• Cardiomyopathy (D)
• Infertility (I &C)
• Renal toxicity (I)
• Cystitis (C)
V: Vincristine, D: Doxorubicin, C: Cyclophosphamide, I: Ifosfamide, E: Etoposide

STUDIES AND TRIALS
Evidence

Induction Chemotherapy
• IESS-I: (VACD) v.s. (VAC) v.s. (VAC +BPR) (Nesbit et al. 1990)
• Localized Ewing sarcoma; N=342
• 5-year RFS: 60% v.s. 24% v.s. 44% (p < 0.001)
• 5-year OS: 65% v.s. 28% v.s. 53% (p < 0.001)
• IESS-II: High-dose intermittent v.s. Moderate-dose
continuous of VACD (Burgert et al. 1990)
• 5-year RFS: 73% v.s. 56% (p=0.03)
• 5-year OS: 77% v.s. 63% (p=0.05)
V: Vincristine; A: Actinomycin D; C: Cyclophosphamide; D: Doxorubicin; BPR: Bilateral pulmonary radiation

Induction Chemotherapy
• INT-0091: (VACD + IE) v.s. VACD (Grier et al. 2003)
• Ewing, PNET or primitive sarcoma of bone; N=398
with non-metastatic dx
• 5-year EFS: 69% v.s. 54% (p=0.005)
• 5-year OS: 72% v.s. 61% (p=0.01)
V: Vincristine; A: Actinomycin D; C: Cyclophosphamide; D: Doxorubicin; I: Ifosfamide; E: Etoposide

Induction Chemotherapy
• INT-0154: VADC/IE in 30 w (dose intensified) v.s. 48 weeks
(Granowetter et al. 2009)
• Ewing sarcoma family of tumors; N=478
• 5-year EFS: 70.1% v.s. 72.1% (p=0.57)
• COG AEWS0031: VDC-IE q2w v.s. q3w (Womer et al. 2012)
• Localized, extradural Ewing sarcoma; N=568
• 5-year EFS: 73% v.s. 65% (p=0.048)
• No increase in toxicity
V: Vincristine; A: Actinomycin D; C: Cyclophosphamide; D: Doxorubicin; I: Ifosfamide; E: Etoposide
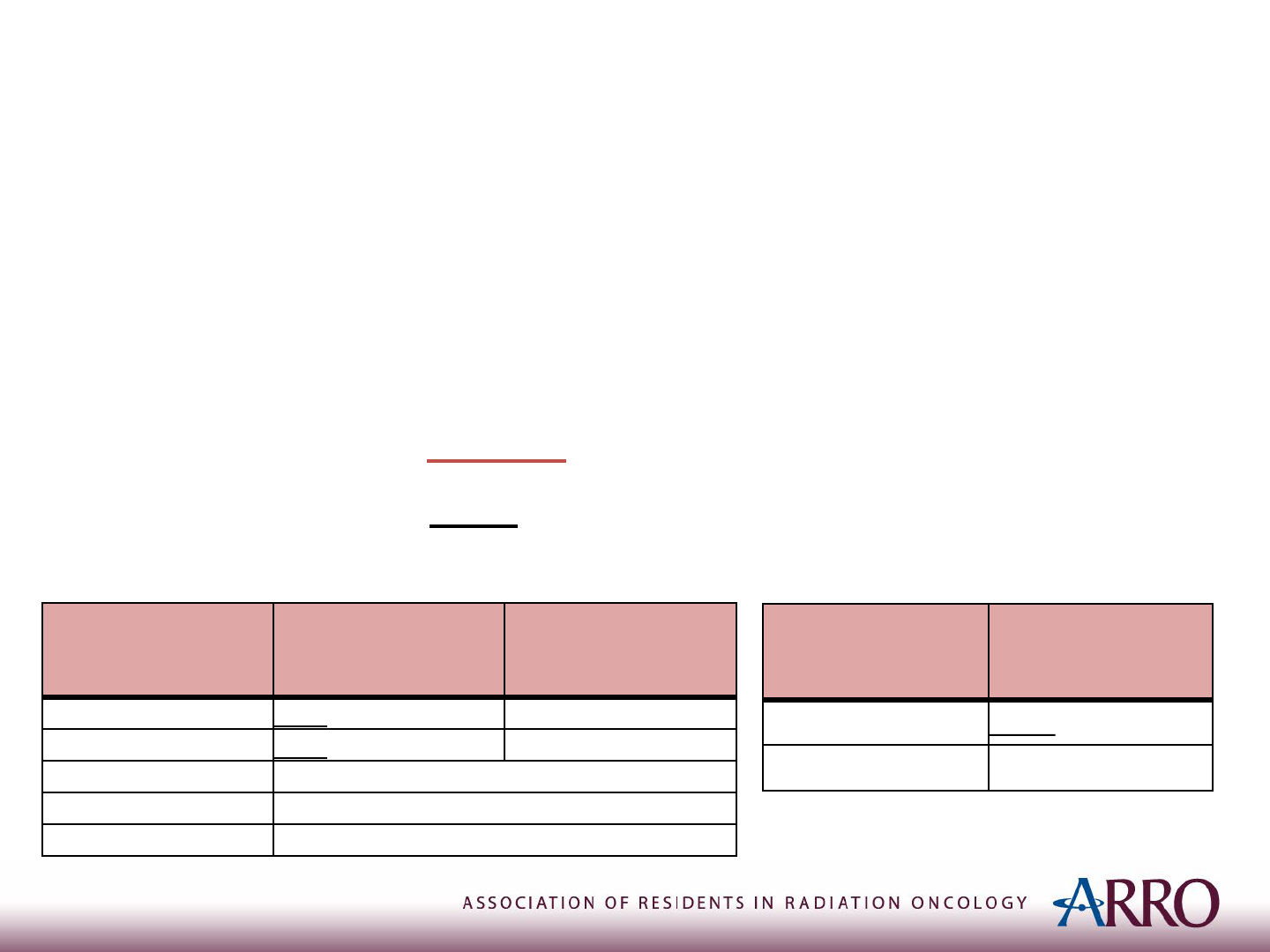
Local Modality: Surgery v.s. RT
• COG Meta Analysis of INT-0091, INT-0154, and AEWS0031
(Ahmed et al. 2017)
• Ewing sarcoma; N=956
• Modality: Surgery v.s. RT v.s. (Surgery + RT)
• 5-year LF: 3.9% v.s. 15.3% (p<0.01) v.s. 6.6% (p=0.12)
• Stratified by tumor location and age:
Location
5-year local failure
Surgery
5-year local failure
Definitive RT
Extremity 3.7%
14.8% (p≦0.01)
Pelvic 3.9%
22.4% (p≦0.01)
Axial non-spine No difference
Spine No difference
Extraskeletal No difference
Age 5-year Local Failure
≧18 years
11.9%
< 18 years 6.7% (p=0.02)
* A statistically greater number of patients who underwent surgery had tumors in more favorable locations (ie. Extremity).

Consolidation Treatment
• Euro-E.W.I.N.G.99 and EWING-2008 (Whelan et al. 2018)
• Localized Ewing sarcoma at high risk for relapse; N=240
• VIDE induction (x6) then
(VAI x1 and BuMel HDT)
v.s. (VAI x 8)
• 8-year EFS: 60.7% v.s. 47.1% (HR of event: 0.64;
p=0.026)
• 8-year OS: 64.5% v.s. 55.6% (HR of death: 0.63;
p=0.028)
V: Vincristine; A: Actinomycin D; D: Doxorubicin; I: Ifosfamide; E: Etoposide; BuMel HDT: High dose Busulfan and Mephalan with autologous SCT
High risk for relapse:
• Poor histologic response (≧10% viable cells) after induction chemotherapy (VIDE)
• Large tumor volume at diagnosis (≧ 200 mL) for tumors that were unresected, initially resected, or resected after radiotherapy

Ewing sarcoma + Pulmonary Mets
• Euro-E.W.I.N.G.99 and EWING-2008 (Dirksen et al. 2019)
• Ewing sarcoma + pulmonary/pleural mets only; N=287
• VIDE induction (x6) then
(VAI x8 with WLI)
v.s. (VAI x1 with BuMel HDT)
• 8-year EFS: 43.1% v.s. 52.9% (HR=0.79, p=0.16)
• No difference in OS (HR=1, p=0.99)
• Toxicity-related death: No patients v.s. 4 patients
V: Vincristine; A: Actinomycin D; D: Doxorubicin; I: Ifosfamide; E: Etoposide; BuMel HDT: High dose Busulfan and Mephalan with autologous SCT
WLI: Whole lung irradiation

Proton therapy & Ewing Sarcoma
• Retrospective chart review (Rombi et al. 2012)
• Pediatric Ewing’s sarcoma at different sites; N=30
• Proton + Chemotherapy
• Median dose: 54 Gy RBE (range: 45-58 Gy)
• 3-year LC, EFS, OS: 86%, 60%, 89% respectively
• Adverse effects:
• Scoliosis/kyphosis (x5)
• Eye canalicular stenosis (x1) & corneal ulcer (x1)
• Endocrine deficiency (x2)
• High frequency hearing loss (x1)
• Secondary hematologic malignancies (x4)

Risk of Secondary Malignancy
• Retrospective chart review (Fuchs et al. 2003)
• Ewing’s sarcoma s/p tx; N=397
• Secondary malignancy (29 tumors) in 26 (6.5%) patients
• Mean interval: 9.5 years (range: 1-32.5 years)
• Distribution:
• Hematologic (x8) - Chemo induced
• Sarcoma (x12) - RT induced
• Carcinoma (x9)
• Worse prognosis in case of sarcoma/hematologic
secondary malignancy

Prospective Trial
• NCT00186992: Radiation Therapy to Treat Musculoskeletal
Tumors
• Phase 2 trial, St. Jude Children's Research Hospital
• Single group assignment, active & not recruiting
• MSK tumors, including Ewing’s; N=202
• Intervention: image-guided radiotherapy
• Outcomes:
• Local control (1
o
)
• RT-related changes in growth and muscle
function

SURVEILLANCE AND RELAPSE
Beyond Treatment

Surveillance
• Physical exam
• CBC
• Imaging
• Intervals
• Initially q2-3 months for at least 2 years
• Annually after 5 years

Relapsed Disease
• 30-40% recurrence
• Very poor prognosis (esp. if within 2 years)
• Management
• Chemotherapy
• +/- RT
• +/- surgery

References
• American Cancer Society. Survival Rates for Ewing Sarcoma. 2022. April 2022. Available from: https://www.cancer.org/cancer/ewing-
tumor/detection-diagnosis-staging/survival-rates.html#references
• Ahmed, S. K., Randall, R. L., DuBois, S. G., Harmsen, W. S., Krailo, M., Marcus, K. J., Janeway, K. A., Geller, D. S., Sorger, J. I., Womer, R. B.,
Granowetter, L., Grier, H. E., Gorlick, R. G., & Laack, N. (2017). Identification of Patients With Localized Ewing Sarcoma at Higher Risk for
Local Failure: A Report From the Children's Oncology Group. International journal of radiation oncology, biology, physics, 99(5), 1286–
1294. https://doi.org/10.1016/j.ijrobp.2017.08.020
• Burgert, E. O., Jr, Nesbit, M. E., Garnsey, L. A., Gehan, E. A., Herrmann, J., Vietti, T. J., Cangir, A., Tefft, M., Evans, R., & Thomas, P. (1990).
Multimodal therapy for the management of nonpelvic, localized Ewing's sarcoma of bone: intergroup study IESS-II. Journal of clinical
oncology : official journal of the American Society of Clinical Oncology, 8(9), 1514–1524. https://doi.org/10.1200/JCO.1990.8.9.1514
• Dirksen U, Brennan B, Deley M-CL, et al. High-Dose Chemotherapy Compared With Standard Chemotherapy and Lung Radiation in Ewing
Sarcoma With Pulmonary Metastases: Results of the European Ewing Tumour Working Initiative of National Groups, 99 Trial and EWING
2008. J Clin Oncol 2019. DOI: https://doi.org/10.1200/JCO.19.00915
• Donaldson, S. S. (2004). Ewing sarcoma: Radiation dose and target volume. Pediatric Blood & Cancer, 41;42;(5;6;), 471-
476. https://doi.org/10.1002/pbc.10472
• Fuchs, B., Valenzuela, R. G., Petersen, I. A., Arndt, C. A., & Sim, F. H. (2003). Ewing's sarcoma and the development of secondary
malignancies. Clinical orthopaedics and related research, (415), 82–89. https://doi.org/10.1097/01.blo.0000093900.12372.e4
• Granowetter, L., Womer, R., Devidas, M., Krailo, M., Wang, C., Bernstein, M., Marina, N., Leavey, P., Gebhardt, M., Healey, J.,
Shamberger, R. C., Goorin, A., Miser, J., Meyer, J., Arndt, C. A., Sailer, S., Marcus, K., Perlman, E., Dickman, P., & Grier, H. E. (2009). Dose-
intensified compared with standard chemotherapy for nonmetastatic Ewing sarcoma family of tumors: a Children's Oncology Group
Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 27(15), 2536–2541.
https://doi.org/10.1200/JCO.2008.19.1478
• Grier HE, Krailo MD, Tarbell NJ, et al. Addition of Ifosfamide and Etoposide to Standard Chemotherapy for Ewing's Sarcoma and Primitive
Neuroectodermal Tumor of Bone. N Engl J Med 2003;348:694-701. DOI: https://doi.org/10.1056/NEJMoa020890

References
• Hornicek, F.J. & Baldini, E.H. (2022). Clinical presentation, staging, and prognostic factors of the Ewing sarcoma family of tumors. In A.S. Pappo, R. Maki, & R.E. Pollock
(Eds.). UpToDate. Available from
https://www-uptodate-com.ezproxy.aub.edu.lb/contents/clinical-presentation-staging-and-prognostic-factors-of-the-ewing-sarcoma-
family-of-tumors?search=ewing%20sarcoma%20staging&source=search_result&selectedTitle=1~97&usage_type=default&display_rank=1#H24919346
• Khanna, N., Pandey, A., & Bajpai, J. (2017). Metastatic Ewing’s sarcoma: Revisiting the “Evidence on the fence”. Indian Journal of Medical and Paediatric
Oncology, 38(2), 173-181. https://doi.org/10.4103/ijmpo.ijmpo_24_17
• Leavey, P. J., Laack, N. N., Krailo, M. D., Buxton, A., Randall, R. L., DuBois, S. G., Reed, D. R., Grier, H. E., Hawkins, D. S., Pawel, B., Nadel, H., Womer, R. B., Letson,
G. D., Bernstein, M., Brown, K., Maciej, A., Chuba, P., Ahmed, A. A., Indelicato, D. J., . . . Mascarenhas, L. (2021). Phase III trial adding vincristine-topotecan-
cyclophosphamide to the initial treatment of patients with nonmetastatic ewing sarcoma: A children's oncology group report. Journal of Clinical Oncology, 39(36), 4029-
4038. https://doi.org/10.1200/JCO.21.00358
• Ludwig, J. A., Meyers, P. A., Dirksen, U., Blay, J., De Pinieux, G., Gouin, F., Riggi, N., Suvà, M. L., & Stamenkovic, I. (2021). Ewing’s sarcoma. The New England Journal
of Medicine, 384(15), 1476-1478. https://doi.org/10.1056/NEJMc2102423
• Mascarenhas, L., Buxton, A., DuBois, S. G., Wang, D., Laack, N. N., Brown, K. L. B., Pawel, B., Nadel, H. R., Davis, J., Hawkins, D. S., Grier, H. E., Womer, R. B.,
Stringham, D., Reed, D. R., Janeway, K. A., Gorlick, R. G., Marina, N., Bernstein, M. L., Krailo, M. D., . . . Bone Sarcoma Committee, Children's Oncology Group. (2020).
Maximum tumor dimension and tumor volume as prognostic factors in patients with newly diagnosed localized Ewing sarcoma (ES)- a report from the Children’s oncology
group (COG). Journal of Clinical Oncology, 38(15_suppl), 11529-11529. https://doi.org/10.1200/JCO.2020.38.15_suppl.11529
• National Comprehensive Cancer Network. “Bone cancer.” Version 2.2022. Accessed: March 2022. Retrieved from
https://www.nccn.org/professionals/physician_gls/pdf/bone.pdf
• National Library of Medicine (U.S.) (2005, April -) Image guided radiotherapy for the treatment of musculoskeletal tumors: a phase II prospective evaluation of radiation-
related treatment effects. Identifier NCT00186992. https://clinicaltrials.gov/ct2/show/NCT00186992
• Nesbit ME Jr, Gehan EA, Burgert EO Jr, et al. Multimodal therapy for the management of primary, nonmetastatic Ewing's sarcoma of bone: a long-term follow-up of the
First Intergroup study. J Clin Oncol 1990;8:1664-74. DOI: https://doi.org/10.1200/JCO.1990.8.10.1664
• Rombi, B., DeLaney, T. F., MacDonald, S. M., Huang, M. S., Ebb, D. H., Liebsch, N. J., Raskin, K. A., Yeap, B. Y., Marcus, K. J., Tarbell, N. J., & Yock, T. I. (2012).
Proton radiotherapy for pediatric Ewing's sarcoma: initial clinical outcomes. International journal of radiation oncology, biology, physics, 82(3), 1142–1148.
https://doi.org/10.1016/j.ijrobp.2011.03.038
• Whelan J, Deley M-CL, Dirksen U, et al. High-Dose Chemotherapy and Blood Autologous Stem-Cell Rescue Compared With Standard Chemotherapy in Localized High-
Risk Ewing Sarcoma: Results of Euro-E.W.I.N.G.99 and Ewing-2008. J Clin Oncol 2018;36:3110-9. DOI: https://doi.org/10.1200/JCO.2018.78.2516
• Womer RB, West DC, Krailo MD, et al. Randomized Controlled Trial of Interval-Compressed Chemotherapy for the Treatment of Localized Ewing Sarcoma: A Report From
the Children's Oncology Group. J Clin Oncol 2012;30:4148-54. DOI: https://doi.org/10.1200/JCO.2011.41.5703
Please provide feedback regarding this case or other ARROcases to arrocas[email protected]
