ConcinN, etal. Int J Gynecol Cancer 2021;31:12–39. doi:10.1136/ijgc-2020-002230
12
ESGO/ESTRO/ESP guidelines for the management
of patients with endometrialcarcinoma
Nicole Concin ,
1,2
Xavier Matias- Guiu,
3,4
Ignace Vergote,
5
David Cibula,
6
Mansoor Raza Mirza,
7
Simone Marnitz,
8
Jonathan Ledermann ,
9
Tjalling Bosse,
10
Cyrus Chargari,
11
Anna Fagotti,
12
Christina Fotopoulou
,
13
Antonio Gonzalez Martin,
14
Sigurd Lax,
15,16
Domenica Lorusso,
12
Christian Marth,
17
Philippe Morice,
18
Remi A Nout,
19
Dearbhaile O'Donnell,
20
Denis Querleu ,
12,21
Maria Rosaria Raspollini,
22
Jalid Sehouli,
23
Alina Sturdza,
24
Alexandra Taylor,
25
Anneke Westermann,
26
Pauline Wimberger,
27
Nicoletta Colombo,
28
François Planchamp,
29
Carien L Creutzberg
30
For numbered afliations see
end of article.
Correspondence to
Nicole Concin, Department of
Gynecology and Obstetrics,
Innsbruck Medical University,
Innsbruck 6020, Austria; nicole.
concin@ i- med. ac. at
For ‘Presented at statement’
see end of article.
Received 7 November 2020
Accepted 16 November 2020
Published Online First
18December2020
To cite: ConcinN,
Matias- GuiuX, VergoteI,
etal. Int J Gynecol Cancer
2021;31:12–39.
Joint statement
© IGCS and ESGO 2021. No
commercial re- use. See rights
and permissions. Published by
BMJ.
Original research
Editorials
Joint statement
Society statement
Meeting summary
Review articles
Consensus statement
Clinical trial
Case study
Video articles
Educational video
lecture
Corners of the world
Commentary
Letters
ijgc.bmj.com
INTERNATIONAL JOURNAL OF
GYNECOLOGICAL CANCER
ABSTRACT
A European consensus conference on endometrial
carcinoma was held in 2014 to produce multi- disciplinary
evidence- based guidelines on selected questions.
Given the large body of literature on the management
of endometrial carcinoma published since 2014, the
European Society of Gynaecological Oncology (ESGO), the
European SocieTy for Radiotherapy and Oncology (ESTRO),
and the European Society of Pathology (ESP) jointly
decided to update these evidence- based guidelines and
to cover new topics in order to improve the quality of care
for women with endometrial carcinoma across Europe and
worldwide.
INTRODUCTION
Endometrial carcinoma is the most common gyneco-
logical cancer in Europe, with a 5- year prevalence of
34.7% (445 805 cases).
1
The estimated number of
new endometrial carcinoma cases in Europe in 2018
was 121 578 with 29 638 deaths, and the incidence
has been rising with aging and increased obesity of
the population. The EUROCARE-5 study, published in
2015, reported a 5- year relative survival of 76% for
European women diagnosed with endometrial carci-
noma in 2000–2007, ranging from 72.9% in Eastern
Europe to 83.2% in Northern Europe.
2
The observed
geographic difference might be partially attributable
to tangible differences in the prevalence of endome-
trioid sub- types among regions. Furthermore, differ-
ences in patient characteristics and histopathologic
features of the disease impact both on patient prog-
nosis and the recommended treatment approach.
A consensus conference including representa-
tion from the European Society of Medical Oncology
(ESMO), the European Society of Gynaecological
Oncology (ESGO), and the European SocieTy for
Radiotherapy and Oncology (ESTRO) was held in
2014 with the aim to produce multi- disciplinary
evidence- based guidelines on 12 selected questions
in order to complement the ESMO clinical practice
guidelines previously published.
3–6
ESGO, ESTRO,
and the European Society of Pathology (ESP) jointly
decided to update these evidence- based guidelines
and, moreover, to cover new topics in order to provide
comprehensive guidelines on all relevant issues of
diagnosis and treatment in endometrial carcinoma
in a multi- disciplinary setting. These guidelines
are intended for use by gynecological oncologists,
general gynecologists, surgeons, radiation oncolo-
gists, pathologists, medical and clinical oncologists,
radiologists, general practitioners, palliative care
teams, and allied health professionals.
RESPONSIBILITIES
These guidelines are a statement of evidence and
consensus of the authors regarding their views of
currently accepted approaches for the management
of patients with endometrial carcinoma. Any clinician
applying or consulting these guidelines is expected
to use independent medical judgment in the context
of individual clinical circumstances to determine any
patient’s care or treatment. These guidelines make no
warranties of any kind regarding their content, use, or
application, and the authors disclaim any responsi-
bility for their application or use in any way.
METHODS
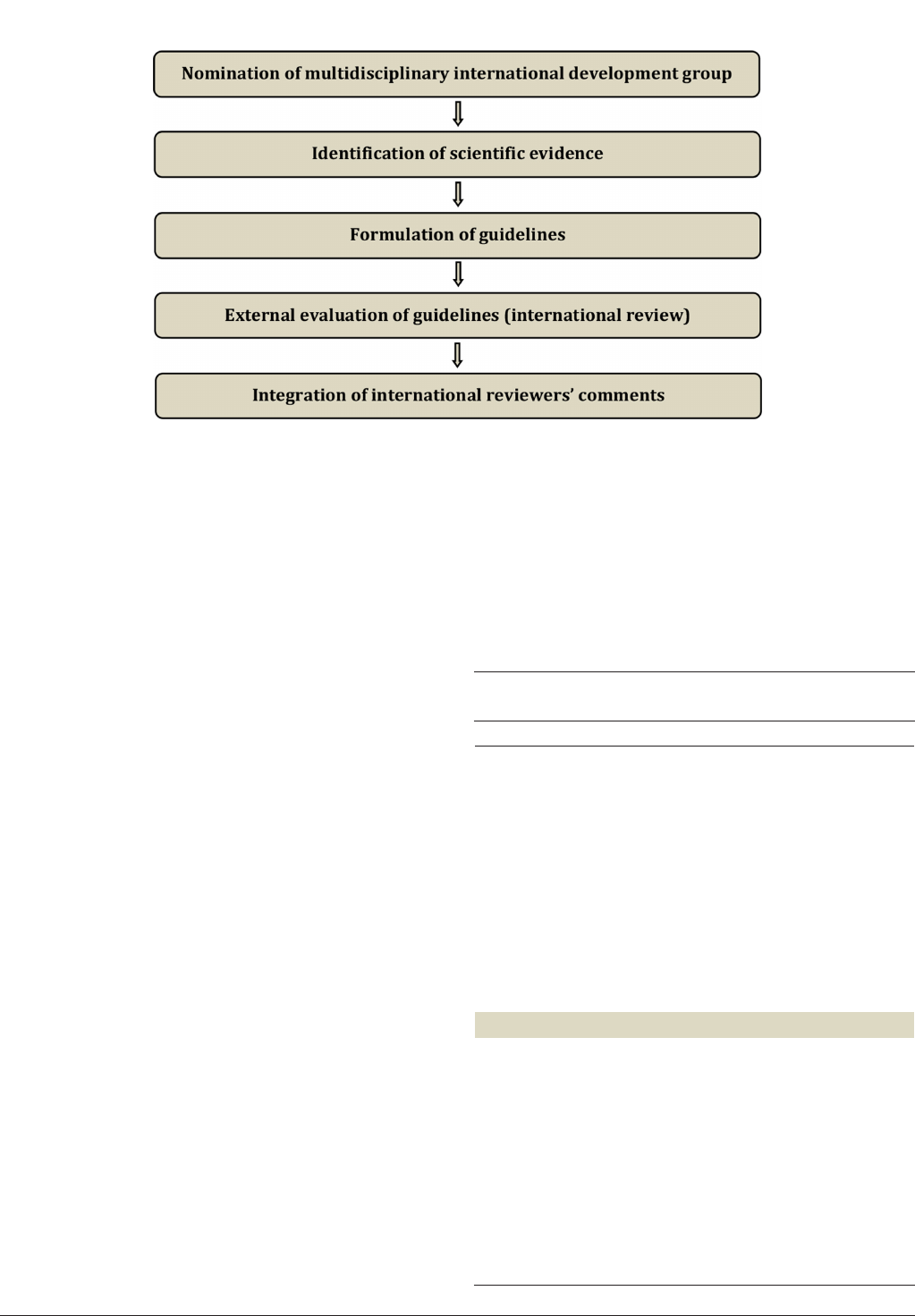
The guidelines were developed using a ve- step
process as dened by the ESGO Guideline Committee
(see Figure1). The strengths of the process include
creation of a multi- disciplinary international devel-
opment group, use of scientic evidence and inter-
national expert consensus to support the guidelines,
and use of an international external review process
(physicians and patients). This development process
involved three meetings of the international devel-
opment group chaired by Professor Nicole Concin
(Medical University of Innsbruck, Innsbruck, Austria/
Evangelische Kliniken Essen- Mitte, Essen, Germany,
for ESGO), Professor Carien L Creutzberg (Leiden
University Medical Center, Leiden, the Nether-
lands, for ESTRO), and Professor Xavier Matias- Guiu
(Department of Pathology, Hospital Universitari Arnau
de Vilanova and Hospital Universitari de Bellvitge,
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2020-002230 on 18 December 2020. Downloaded from
13
ConcinN, etal. Int J Gynecol Cancer 2021;31:12–39. doi:10.1136/ijgc-2020-002230
Joint statement
Irblleida, Idibell, Universities of Lleida and Barcelona, CIBERONC,
Spain, for ESP).
ESGO/ESTRO/ESP nominated practising clinicians who are
involved in the management of patients with endometrial carcinoma
and have demonstrated leadership in the clinical management of
patients through research, administrative responsibilities, and/or
committee membership to serve on the expert panel. The objective
was to assemble a multi- disciplinary panel and it was therefore
essential to include professionals from relevant disciplines (gyne-
cological oncology and gynecology, medical, clinical and radiation
oncology, pathology) to contribute to the validity and acceptability of
the guidelines. To ensure that the statements were evidence based,
the current literature was reviewed and critically appraised. A
systematic literature review of relevant studies published between
January 2014 and June 2019 was carried out using the MEDLINE
database (see online supplemental appendix 1). The literature
search was limited to publications in English. Priority was given to
high- quality systematic reviews, meta- analyses, and randomized
controlled trials, but studies of lower levels of evidence were also
evaluated. The search strategy excluded editorials, letters, and in
vitro studies. The reference list of each identied article was also
reviewed for other potentially relevant articles.
The development group developed guidelines for all the topics.
The guidelines were retained if they were supported by a suf-
ciently high level of scientic evidence and/or when a large
consensus among experts was obtained. An adapted version of the
'Infectious Diseases Society of America- United States Public Health
Service Grading System' was used to dene the level of evidence
and grade of recommendation for each of the recommendations
7
(see Table1). In the absence of any clear scientic evidence, judg-
ment was based on the professional experience and consensus of
the development group.
ESGO/ESTRO/ESP established a large multi- disciplinary panel
of practicing clinicians who provide care to patients with endo-
metrial carcinoma to act as independent expert reviewers for the
guidelines developed. These reviewers were selected according to
their expertise, had to be still involved in clinical practice, and were
from different European and non- European countries to ensure
global perspective. Patients with endometrial carcinoma were also
included. These independent reviewers were asked to evaluate
each recommendation according to its relevance and feasibility in
clinical practice (only physicians), so that comprehensive quantita-
tive and qualitative evaluations of the guidelines were completed.
Patients were asked to evaluate qualitatively each recommen-
dation (according to their experience, personal perceptions, etc).
Figure 1 Development process.
Table 1 Levels of evidence and grades of
recommendations
Levels of evidence
I Evidence from at least one large randomized
controlled trial of good methodological quality
(low potential for bias) or meta- analyses of well-
conducted randomized trials without heterogeneity
II Small randomized trials or large randomized trials
with a suspicion of bias (lower methodological
quality) or meta- analyses of such trials or of trials
with demonstrated heterogeneity
III Prospective cohort studies
IV Retrospective cohort studies or case–control studies
V Studies without control group, case reports, expert
opinions
Grades of recommendations
A Strong evidence for efcacy with a substantial clinical
benet, strongly recommended
B Strong or moderate evidence for efcacy but with a
limited clinical benet, generally recommended
C Insufcient evidence for efcacy or benet does not
outweigh the risk or the disadvantages (adverse
events, costs, etc), optional
D Moderate evidence against efcacy or for adverse
outcome, generally not recommended
E Strong evidence against efcacy or for adverse
outcome, never recommended
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2020-002230 on 18 December 2020. Downloaded from

14
ConcinN, etal. Int J Gynecol Cancer 2021;31:12–39. doi:10.1136/ijgc-2020-002230
Joint statement
Evaluations of the external reviewers (n=191) were pooled and
discussed by the international development group before nalising
the guidelines. The list of the 191 external reviewers is available in
online supplemental appendix 2.
GENERAL RECOMMENDATIONS
► Planning of staging and treatment should be made on a
multi- disciplinary basis (generally at a tumor board meeting,
composed according to local guidelines) and based on the
comprehensive and precise knowledge of prognostic and
predictive factors for outcome, morbidity, and quality of life (V,
A).
► Patients should be carefully counseled about the suggested
diagnostic and treatment plan and potential alternatives,
including risks and benets of all options (V, A).
► Treatment should be undertaken in a specialized center by a
dedicated team of specialists in the diagnosis and manage-
ment of gynecological cancers, especially in high- risk and/or
advanced stage disease (V, A).
IDENTIFICATION AND SURVEILLANCE OF WOMEN WITH A
PATHOGENIC GERMLINE VARIANT IN A LYNCH SYNDROME-
ASSOCIATED GENE
Approximately 3% of all endometrial carcinomas and about 10%
of mismatch repair decient (MMRd)/microsatellite unstable endo-
metrial carcinomas are causally related to germline mutations of
one of the MMR genes MLH1, PMS2, MSH2 and MSH6.
8
Testing
for MMR status/microsatellite instability (MSI) in endometrial carci-
noma patients has been shown to be relevant for four reasons: (1)
diagnostic, as MMRd/MSI is considered a marker for endometrioid-
type endometrial carcinoma; (2) pre- screening to identify patients at
higher risk for having Lynch syndrome; (3) prognostic, as identied
by The Cancer Genome Atlas (TCGA, see below for molecular clas-
sication); and (4) predictive for potential utility of immune check-
point inhibitor therapy. The International Society of Gynecological
Pathology (ISGyP) has recommended testing for MMR status/MSI in
all endometrial carcinoma samples, irrespective of age.
9
This has
also been recommended in other society statements and recom-
mendations, such as the Manchester International Consensus
Group recommendations, whenever resources are available.
10
The preferred approach (widely available and cost- effective) to
identifying patients with a higher chance of having Lynch syndrome
is by MMR- immunohistochemistry (IHC) on well preserved tumor
tissue. MMR- IHC is a reliable method to assess MMR status, and
in addition provides information on the altered gene/protein. ISGyP
guidelines therefore recommend MMR- IHC as the preferred test.
9
MMR- IHC consists of the assessment of the expression of four
MMR proteins (MLH1, PMS2, MSH6, and MSH2). A simplied two-
antibody (PMS2 and MSH6) approach has been proposed as a cost-
effective alternative.
11–13
This procedure still requires performing
MLH1 and MSH2 IHC in cases with any abnormal staining of PMS2
and/or MSH6. Molecular analyses for the microsatellite status
(MSI- test) are an alternative, but are more laborious, require non-
neoplastic tissue, are more expensive, and do not provide infor-
mation on the gene affected. For optimal pre- selection of patients
at risk for having Lynch syndrome, both approaches require the
analysis of MLH1 promoter methylation status in cases with loss of
MLH1/PMS2 expression. Testing for MMRd by IHC or MSI by PCR-
based methods does not allow direct identication of patients with
Lynch syndrome since MMRd/MSI is frequently due to sporadic
events such as bi- allelic somatic mutations or hypermethylation.
In the absence of hypermethylation, referral to genetic counseling
is recommended to evaluate the presence of a germline muta-
tion. When familial history is highly suspicious of Lynch syndrome,
genetic counseling is recommended independent of the MMR
status.
The cumulative incidences for cancer depend on the specic
mutation in women with Lynch sydrome. For endometrial carci-
noma, the cumulative incidences at 70 years are 34%, 51%,
49%, and 24% for MLH1, MSH2, MSH6, and PMS2 mutation
carriers, respectively, and for ovarian cancer 11%, 15%, 0%,
and 0%, respectively.
14
Furthermore, the age of cancer onset in
Lynch syndrome varies among specic mutated genes and types
of mutations.
15
Ryan et al suggest gynecological surveillance to
be appropriate from age 30 years for those with MSH2 mutations,
from age 35 years for those with nontruncating MLH1 mutations,
and from age 40 years for those with MSH6 and truncating MLH1
mutations. Women with heterozygous PMS2 mutations do not
warrant gynecological surveillance because their absolute risk of
gynecological cancer is very low. As part of a retrospective study,
Lachiewicz et al reported a risk of any occult malignancy during
prophylactic surgery for women with Lynch syndrome or heredi-
tary non- polyposis colorectal cancer to be up to 17%.
16
Thus, these
patients should be counseled about the risk of detection of gyneco-
logical cancer at prophylactic surgery.
Recommendations
► To identify patients with Lynch syndrome and triage for germline
mutational analysis, MMR IHC (plus analysis of MLH1 promotor
methylation status in case of immunohistochemical loss of
MLH1/PMS2 expression) or MSI tests should be performed in
all endometrial carcinomas, irrespective of histologic subtype
of the tumor (III, B).
► Endometrial carcinoma patients identied as having an
increased risk of Lynch syndrome should be offered genetic
counseling (III, B).
► Surveillance for endometrial carcinoma in Lynch syndrome
mutation carriers should in general start at the age of 35 years;
however, individual factors need to be taken into consideration
(tailored surveillance programs). The decision on the starting
age of surveillance should integrate knowledge on the specic
mutation and history of onset of events in the family (IV, B).
► Surveillance of the endometrium by annual transvaginal ultra-
sound (TVUS) and annual or biennial biopsy until hysterectomy
should be considered in all Lynch syndrome mutation carriers
(IV, B).
► Hysterectomy and bilateral salpingo- oophorectomy to prevent
endometrial and ovarian cancer should be performed at the
completion of childbearing and preferably before the age of
40 years. All the pros and cons of prophylactic surgery must
be discussed including the risk of occult gynecological cancer
detection at prophylactic surgery. Estrogen replacement
therapy should be suggested if bilateral salpingo- oophorectomy
is performed in pre- menopausal women (IV, B).
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2020-002230 on 18 December 2020. Downloaded from

15
ConcinN, etal. Int J Gynecol Cancer 2021;31:12–39. doi:10.1136/ijgc-2020-002230
Joint statement
MOLECULAR MARKERS FOR ENDOMETRIAL CARCINOMA
DIAGNOSIS AND AS DETERMINANTS FOR TREATMENT
DECISIONS
Different types of endometrial carcinoma have specic histolog-
ical and molecular features, precursor lesions and natural histo-
ries. Conventional pathologic analysis remains an important tool
for tumor stratication, but suffers from inter- observer varia-
tion. Different groups have applied a diagnostic algorithm using
three immunohistochemical markers (p53, MSH6 and PMS2)
and one molecular test (mutation analysis of the exonuclease
domain of POLE) to identify prognostic groups analogous to the
TCGA molecular- based classication.
17–21
The feasibility of this
approach was conrmed by a large number of publications that
have all consistently reported prognostic relevance particularly in
high- grade and high- risk tumors in several independent cohorts
and prospective clinical trials.
22
To apply this molecular classica-
tion, all these diagnostic tests need to be performed. Performing
one of the surrogate marker tests in isolation is insufcient, as a
combination of positive tests can occur in approximatively 5% of
all carcinomas. The diagnostic algorithm to classify these so- called
'multiple classiers' has been described recently.
23 24
In addition,
endometrial carcinoma should only be classied as POLE- mutated
(POLEmut) when pathogenic variants of POLE are identied in the
gene’s exonuclease domain.
25 26
This surrogate marker approach to the molecular- based classi-
cation has been demonstrated to be prognostically informative in
low-, intermediate-, and high- risk endometrial carcinoma. Smaller
studies showed that the molecular classication is also applicable
to non- endometrioid tumors including serous, clear cell, undiffer-
entiated carcinomas, and uterine carcinosarcomas. For adjuvant
treatment recommendations, the molecular classication seems to
be particularly relevant in the context of high- grade and/or high- risk
endometrial carcinomas. Application of the molecular classication
in high- grade and/or high- risk endometrial carcinomas shows that
there is a group of patients with an excellent prognosis—that is,
the POLEmut tumors—and a group with a poor prognosis—that is,
the p53- abnormal (p53abn) tumors. Endometrial carcinomas with
MMRd or non- specic molecular prole (NSMP) have an interme-
diate prognosis. However, the molecular surrogate is not perfect.
Immunohistochemical demonstration of p53abn is a good but not
perfect surrogate of TP53 mutation. Furthermore, a small propor-
tion of high copy number tumors do not show TP53 mutations. To
minimize these limitations, an integrated analysis combining tradi-
tional pathologic and molecular results seems ideal. In low- risk
endometrioid carcinomas, the molecular classication may not be
required.
27 28
The proposed molecular classication of endometrial carcinoma
is clinically feasible using a limited set of diagnostic tests. Using
this novel classication is encouraged. All diagnostic tests should
be performed in conjunction due to the occurrence of 'double clas-
siers'.
23
Clinical management may be particularly impacted by the
molecular classication in scenarios where adjuvant chemotherapy
is considered (high- grade/high- risk disease). Thus, these cases
should be prioritized when there is a lack of sufcient resources to
perform this classication on all endometrial carcinomas. If molec-
ular classication tools are not available, endometrial carcinoma
classication should be based on traditional pathologic features.
There is still room for other biomarkers that may be potentially
useful in the big group of low- grade endometrioid carcinoma with
NSMP, such as L1CAM expression or mutations in CTNNB1.
29–32
Recommendations
► Molecular classication is encouraged in all endometrial carci-
nomas, especially high- grade tumors (IV, B).
► POLE mutation analysis may be omitted in low- risk and
intermediate- risk endometrial carcinoma with low- grade
histology (IV, C).
DEFINITION OF PROGNOSTIC RISK GROUPS INTEGRATING
MOLECULAR MARKERS
There is overwhelming evidence that traditional pathologic features,
such as histopathologic type, grade, myometrial invasion, and
lymphovascular space invasion (LVSI), are important in assessing
prognosis, as recommended in the ISGyP guidelines.
9
Histopatho-
logic typing should be performed according to the WHO Classica-
tion of Tumors (5th edition).
33
A binary International Federation of
Gynecology and Obstetrics (FIGO) grading is recommended, which
considers grade 1 and grade 2 carcinomas as low- grade and grade
3 carcinomas as high- grade. For the assessment of myometrial
invasion, account needs to be taken of the endo- myometrial junc-
tion which is undulating.
34
Focal LVSI is dened by the presence
of a single focus around the tumor, substantial LVSI as multifocal
or diffuse arrangement of LVSI or the presence of tumor cells in
ve or more lymphovascular spaces. The molecular classication
adds another layer of information to the conventional morphologic
features and therefore should be integrated in the pathologic report.
Recommendations
► Histopathologic type, grade, myometrial invasion, and LVSI (no/
focal/substantial) should be recorded in all patients with endo-
metrial carcinoma (V, A).
► The denition of prognostic risk groups is presented in Table2
for both situations when molecular classication is known or
unknown.
PRE- AND INTRA-OPERATIVE WORK-UP
Risk group allocation on biopsy according to the WHO Classication
of Tumors (5th edition) and FIGO grading of endometrial carcinoma
is required for adequate planning of therapy.
33
Histopathologic
grade has prognostic relevance. A modied binary FIGO grading is
recommended lumping together grade 1 and grade 2 endometrioid
carcinomas as low- grade and grade 3 as high- grade.
Magnetic resonance imaging (MRI) techniques are highly specic
in the assessment of deep myometrial invasion, cervical stromal
involvement, and lymph node metastasis.
35–82
The diagnostic
performance of TVUS and MRI for detecting myometrial invasion
in endometrial carcinoma are quite similar.
39 44 56 83–88
Of note, pre-
operative ultrasound assessment of deep myometrial and cervical
stromal invasion in endometrial carcinoma is best performed by an
expert sonographer as, compared with gynecologists, they show
a greater degree of agreement with histopathology and greater
inter- observer reproducibility.
84
Positron emission tomography
(PET) scanning has an excellent specicity for the pre- operative
assessment of lymph node metastases in patients with endome-
trial carcinoma. Its moderate sensitivity for detecting lymph node
metastases during preo- perative staging probably reects the need
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2020-002230 on 18 December 2020. Downloaded from
16
ConcinN, etal. Int J Gynecol Cancer 2021;31:12–39. doi:10.1136/ijgc-2020-002230
Joint statement
for a sufcient number of neoplastic cells to induce
18
F- uoro-
2- deoxy- D- glucose hypermetabolism.
89–100
The usefulness of
maximal standardized uptake value in classifying patients into pre-
dened risk groups is limited.
101
A pre- operative CT scan has a
clinical utility in patients with endometrial carcinoma in detecting
metastatic disease.
102 103
Frozen section of endometrial biopsy material is obsolete.
Myometrial invasion should not be assessed by frozen section
because of poor reproducibility and agreement with denitive
parafn sections. Since sentinel node biopsy is increasingly used,
the need for intra- operative assessment of myometrial invasion has
become less important. Moreover, some of the biomarkers that have
been proposed require optimal management of the surgical spec-
imen with high quality pre- analytical issues such as appropriate
xation conditions. Performing frozen sections can lead to incor-
rect control of pre- analytical conditions, sometimes even leading
to incorrect assessment of LVSI due to artifactual displacement of
tumor cells into vascular spaces during processing. In addition, the
freezing of tissue before xation and further processing interferes
with an optimal pre- analytical procedure required for standardized
histopathologic diagnosis.
Recommendations
► Histopathologic tumor type and grade in endometrial biopsy is
required (IV, A).
► Pre- operative mandatory work- up includes: family history;
general assessment and inventory of co- morbidities; geriatric
assessment, if appropriate; clinical examination, including
pelvic examination; expert transvaginal or transrectal ultra-
sound or pelvic MRI (IV, C).
► Depending on clinical and pathologic risk, additional imaging
modalities (thoracic, abdominal and pelvic CT scan, MRI, PET
scan, or ultrasound) should be considered to assess ovarian,
nodal, peritoneal, and other sites of metastatic disease (IV, C).
Table 2 Denition of prognostic risk groups
Risk group Molecular classication unknown Molecular classication known*†
Low
► Stage IA endometrioid + low- grade‡ +
LVSI negative or focal
► Stage I–II POLEmut endometrial carcinoma,
no residual disease
► Stage IA MMRd/NSMP endometrioid
carcinoma + low- grade‡ + LVSI negative or focal
Intermediate
► Stage IB endometrioid + low- grade‡ +
LVSI negative or focal
► Stage IA endometrioid + high- grade‡ +
LVSI negative or focal
► Stage IA non- endometrioid (serous,
clear cell, undifferentiared carcinoma,
carcinosarcoma, mixed) without myometrial
invasion
► Stage IB MMRd/NSMP endometrioid
carcinoma + low- grade‡ + LVSI negative or focal
► Stage IA MMRd/NSMP endometrioid
carcinoma + high- grade‡ + LVSI negative or
focal
► Stage IA p53abn and/or non- endometrioid
(serous, clear cell, undifferentiated carcinoma,
carcinosarcoma, mixed) without myometrial
invasion
High–intermediate
► Stage I endometrioid + substantial LVSI
regardless of grade and depth of invasion
► Stage IB endometrioid high- grade‡
regardless of LVSI status
► Stage II
► Stage I MMRd/NSMP endometrioid
carcinoma + substantial LVSI regardless of grade
and depth of invasion
► Stage IB MMRd/NSMP endometrioid
carcinoma high- grade‡ regardless of LVSI status
► Stage II MMRd/NSMP endometrioid
carcinoma
High
► Stage III–IVA with no residual disease
► Stage I–IVA non- endometrioid (serous,
clear cell, undifferentiated carcinoma,
carcinosarcoma, mixed) with myometrial
invasion, and with no residual disease
► Stage III–IVA MMRd/NSMP endometrioid
carcinoma with no residual disease
► Stage I–IVA p53abn endometrial carcinoma
with myometrial invasion, with no residual
disease
► Stage I–IVA NSMP/MMRd serous,
undifferentiated carcinoma, carcinosarcoma with
myometrial invasion, with no residual disease
Advanced
metastatic
► Stage III–IVA with residual disease
► Stage IVB
► Stage III–IVA with residual disease of any
molecular type
► Stage IVB of any molecular type
*For stage III–IVA POLEmut endometrial carcinoma and stage I–IVA MMRd or NSMP clear cell carcinoma with myometrial invasion,
insufcient data are available to allocate these patients to a prognostic risk group in the molecular classication. Prospective registries are
recommended.
†See text on how to assign double classiers (eg, patients with both POLEmut and p53abn should be managed as POLEmut).
‡According to the binary FIGO grading, grade 1 and grade 2 carcinomas are considered as low- grade and grade 3 carcinomas are
considered as high- grade.
LVSI, lymphovascular space invasion; MMRd, mismatch repair decient; NSMP, non- specic molecular prole; p53abn, p53 abnormal;
POLEmut, polymerase- mutated.
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2020-002230 on 18 December 2020. Downloaded from

17
ConcinN, etal. Int J Gynecol Cancer 2021;31:12–39. doi:10.1136/ijgc-2020-002230
Joint statement
► Intra- operative frozen section is not encouraged for myometrial
invasion assessment because of poor reproducibility and inter-
ference with adequate pathologic processing (IV, A).
EARLY STAGE DISEASE
Surgical management of apparent stage I/II endometrial
carcinomas
Minimally invasive approach
Two randomized prospective studies comparing minimally invasive
with open surgeries showed similar survival with quicker recovery
with the minimally invasive approach.
104 105
More recently, pooled
analyses of randomized prospective studies including, notably, these
two studies and multiple retrospective and prospective studies also
support the use of minimally invasive surgery for patients including
those with high- risk endometrial carcinoma.
106–171
Recommendations
► Minimally invasive surgery is the preferred surgical approach,
including patients with high- risk endometrial carcinoma (I, A).
► Any intra- peritoneal tumor spillage, including tumor rupture or
morcellation (including in a bag), should be avoided (III, B).
► If vaginal extraction risks uterine rupture, other measures
should be taken (eg, mini- laparotomy, use of endobag) (III, B).
► Tumors with metastases outside the uterus and cervix
(excluding lymph node metastases) are relative contra-
indications for minimally invasive surgery (III, B).
Standard surgical procedures
In a randomized controlled trial comparing modied radical (Piver–
Rutledge class II) hysterectomy to the standard extrafascial (Piver–
Rutledge class I) or simple total hysterectomy in stage I endometrial
carcinoma, Signorelli et al showed no differences in locoregional
control and survival.
172
The high risk of microscopic omental metas-
tases in stage I serous and undifferentiated endometrial carcinoma
and in carcinosarcoma suggests that omentectomy should be part
of staging surgery in these patients.
173
The low rate of omental
metastases in apparent clinical stage I endometrioid and clear cell
carcinoma does not justify the procedure.
174
Although the risk of
having occult (microscopic) omental metastases in carcinosarcoma
is around 6%, staging omentectomy in these women is suggested.
Identication of these cases will allow inclusion of patients with
advanced stage disease into clinical trials.
175
Positive peritoneal
cytology correlates with poor prognostic factors and poor survival;
however, it is not part of FIGO staging and unclear if this should
inuence treatment decisions.
176–178
Recommendations
► Standard surgery is total hysterectomy with bilateral salpingo-
oophorectomy without vaginal cuff resection (II, A).
► Staging infracolic omentectomy should be performed in clinical
stage I serous endometrial carcinoma, carcinosarcoma, and
undifferentiated carcinoma. It can be omitted in clear cell and
endometrioid carcinoma in stage I disease (IV, B).
► Surgical re- staging can be considered in previously incom-
pletely staged patients with high– intermediate- risk/high- risk
disease if the outcome might have an implication for adjuvant
treatment strategy (IV, B).
Lymph node staging
Sentinel node biopsy has been introduced as an alternative to lymph
node dissection for lymph node staging and, if done according to
state- of- art principles, a negative sentinel node is accepted to
conrm pN0. Multiple studies, including prospective cohort ones,
conrmed high sensitivity of sentinel lymph node status for lymph
node staging in patients with early- stage endometrial carcinoma
and support the impact of sentinel lymph node biopsy on surgical
management and indications for adjuvant therapies.
179–241
More
intensive pathologic assessment of sentinel lymph node (sentinel
lymph node ultrastaging) supports the detection of small metas-
tases which could be missed by standard evaluation.
214 232
Sentinel
lymph node biopsy without dissection of other pelvic lymph nodes is
associated with subtantially lower risk of post- operative morbidity,
especially lower leg lymphedema.
242
In a large group of patients
with low- risk (myometrial invasion <50%, low- grade) endometrial
carcinoma with sentinel lymph node biopsy, lymph node involve-
ment was found in 6% of patients, half of them identied by patho-
logic ultrastaging.
243
Patients with tumors without myometrial inva-
sion did not have any positive sentinel lymph nodes. Four prospec-
tive cohort trials have shown high sensitivity to detect pelvic lymph
node metastases and a high negative predictive value by applying
a sentinel lymph node algorithm in high- risk/high- grade endome-
trial carcinomas in the hands of experienced surgeons.
181 182 237 244
Recently, a randomized controlled trial highlighted that the use
of indocyanine green instead of methylene blue dye resulted in
a signicant increase in sentinel lymph node detection rates per
hemipelvis in women with endometrial carcinoma undergoing mini-
mally invasive surgery.
245
Retrospective studies showed a similar
prognosis for patients after full lymphadenectomy and sentinel
lymph node biopsy only.
179 201 220
High bilateral pelvic sentinel
lymph node detection can be achieved when the tracer is injected
into the cervix.
180 246
A higher sentinel lymph node detection rate
has been reported using near- infrared uorescence in comparison
to other techniques.
247
A worse prognosis is associated with the
presence of nodal micrometastases, especially in patients who do
not receive adjuvant treatment.
248
There is no evidence that the
presence of isolated tumor cells (ITCs) has an impact on prognosis,
and similar to other tumor sites, the stage would be pN0(i+). If
pelvic lymph node involvement is reported either by sentinel lymph
node frozen section or by the nal pathology, para- aortic staging
can be considered, either by imaging (with all limitations of the
imaging modalities) or by surgery. It should be noted that, based on
data from two large randomized trials, lymph node staging does not
have a therapeutic value but is done to assess the extent of disease
and to provide information for adjuvant treatment decisions.
249 250
Frozen section on specimens regarded as sentinel lymph nodes
can conrm the presence of lymph nodes and macrometastases
but should not replace adequate pathologic processing and ultrast-
aging.
Recommendations
► Sentinel lymph node biopsy can be considered for staging
purposes in patients with low- risk/intermediate- risk disease. It
can be omitted in cases without myometrial invasion. System-
atic lymphadenectomy is not recommended in this group (II, A).
► Surgical lymph node staging should be performed in patients
with high–intermediate- risk/high- risk disease. Sentinel
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2020-002230 on 18 December 2020. Downloaded from

18
ConcinN, etal. Int J Gynecol Cancer 2021;31:12–39. doi:10.1136/ijgc-2020-002230
Joint statement
lymph node biopsy is an acceptable alternative to systematic
lymphadenectomy for lymph node staging in stage I/II (III, B).
► If sentinel lymph node biopsy is performed (II, A):
– Indocyanine green with cervical injection is the preferred
detection technique.
– Tracer re- injection is an option if sentinel lymph node is not
visualized upfront.
– Side- specic systematic lymphadenectomy should be per-
formed in high–intermediate- risk/high- risk patients if senti-
nel lymph node is not detected on either pelvic side.
– Pathologic ultrastaging of sentinel lymph nodes is
recommended.
► When a systematic lymphadenectomy is performed, pelvic and
para- aortic infrarenal lymph node dissection is suggested (III,
B).
► Presence of both macrometastases and micrometastases
(<2 mm, pN1(mi)) is regarded as a metastatic involvement (IV,
C).
► The prognostic signicance of ITCs, pN0(i+), is still uncertain
(IV, C).
► If pelvic lymph node involvement is found intra- operatively,
further systematic pelvic lymph node dissection should be
omitted. However, debulking of enlarged lymph nodes and
para- aortic staging can be considered (IV, B).
Option for ovarian preservation and salpingectomy in stage I/II
A meta- analysis showed that there was no signicant difference
in overall survival between patients treated with ovarian preser-
vation and bilateral salpingo- oophorectomy.
251
A similar result
was achieved in young and pre- menopausal women. Disease- free
survival of patients whose ovaries were preserved was slightly
compromised, but this was not statistically signicant. Ovarian
preservation can be cautiously considered in specic clinical situa-
tions when treating young and pre- menopausal women with early
stage endometrial carcinoma because it is not associated with a
signicant adverse impact on survival.
252–254
Salpingectomy during
hysterectomy is recommended to decrease the risk of high- grade
serous ovarian carcinoma.
255
Ovarian preservation is not recom-
mended in patients with cancer family history involving ovarian
cancer risk (eg, BRCA mutation, Lynch syndrome, etc), but oocyte
cryopreservation might be considered.
256
Recommendations
► Ovarian preservation can be considered in pre- menopausal
patients aged <45 years with low- grade endometrioid endo-
metrial carcinoma with myometrial invasion <50% and no
obvious ovarian or other extra- uterine disease (IV, A).
► In cases of ovarian preservation, salpingectomy is recom-
mended (IV, B).
► Ovarian preservation is not recommended for patients with
cancer family history involving ovarian cancer risk (eg, BRCA
mutation, Lynch syndrome, etc) (IV, B).
Radicality of surgery for clinical stage II
Radicality of hysterectomy (simple vs modied radical hysterec-
tomy (type II)) in stage I–III endometrial carcinoma has no impact
on local recurrence rate, disease- free survival, and overall survival.
In a meta- analysis enrolling 2866 patients with stage II endometrial
carcinoma, radical hysterectomy did not show a signicant survival
benet for either overall survival or progression- free survival
compared with simple hysterectomy.
257
The result remained
consistent after it was adjusted for the possible impact of adjuvant
radiotherapy.
Recommendations
► Total hysterectomy with bilateral salpingo- oophorectomy and
lymph node staging is the surgical standard of care in patients
with stage II endometrial carcinoma (IV, B).
► More extensive procedures should only be performed if required
to achieve free surgical margins (IV, B).
Medically unt patients
It is rare for patients to be unt for surgery, but medical co- morbidi-
ties, which increasingly include morbid obesity, can preclude surgery
due to high operative and peri- operative risks. Ideally, assessment
should be undertaken in a center with specialist anesthetic experi-
ence in managing these high- risk patients. Denitive radiotherapy
with brachytherapy, external beam radiation therapy (EBRT) or the
combination of both modalities can be considered.
258–262
Recommendations
► Medical contra- indications to the standard surgical manage-
ment by minimally invasive surgery are rare. Vaginal hyster-
ectomy, with bilateral salpingo- oophorectomy if feasible, can
be considered in patients unt for the recommended standard
surgical therapy (IV, C).
► Denitive radiotherapy can be considered for primary tumors
where surgery is contra- indicated for medical reasons:
– The combination of EBRT and brachytherapy should be used
for high- grade tumors and/or deep myometrial invasion (II,
B).
– For low- grade tumors, brachytherapy alone can be consid-
ered (II, B).
– In medically unt patients unsuitable for curative surgery or
radiotherapy, systemic treatment (including hormonal ther-
apy) can be considered (IV, B).
Fertility preservation
Work-up for fertility preservation treatments
Fertility- sparing treatments should be considered in patients with
atypical hyperplasia/endometrioid intra- epithelial neoplasia (AH/
EIN) or grade 1 endometrioid carcinoma without myometrial inva-
sion.
263–269
There are very few published data on patients with
stage IA grade 2 endometrioid carcinoma without myometrial
invasion who received fertility- sparing treatment with combined
oral medroxyprogesterone acetate/levonorgestrel intrauterine
system.
270
Although results are encouraging, this treatment should
only be considered by experienced gynecological oncologists using
well- dened protocols with detailed patient information and close
follow- up.
Hysteroscopic biopsy is suggested, based on its higher agree-
ment with the nal diagnosis compared with dilatation and curet-
tage.
271 272
Although hysteroscopy seems to be associated with a
higher rate of positive peritoneal cytology, it seems not to have a
negative impact on survival.
273
Expert vaginal ultrasound examina-
tion can be used instead of pelvic MRI. Its high diagnostic perfor-
mance allows the detection of myometrial invasion and cervical
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2020-002230 on 18 December 2020. Downloaded from

19
ConcinN, etal. Int J Gynecol Cancer 2021;31:12–39. doi:10.1136/ijgc-2020-002230
Joint statement
stromal invasion with respect to nal pathologic examination.
Ultrasound should be performed by an expert sonographer (a prac-
titioner who spends a signicant part of her/his time undertaking
ultrasound examinations in gynecology and gynecologic oncology
and has fullled the minimum training requirements for level 3
following the recommendations of the European Federation of Soci-
eties for Ultrasound in Medicine and Biology).
274
There is currently a lack of high- quality evidence regarding the
correlation between weight loss and reduction of risk of recurrence/
increased survival in patients with endometrial carcinoma, espe-
cially with respect to fertility- sparing treatment.
275
Diabetes mellitus
does not seem to affect the outcome of conservative treatment in
women with AH/EIN or early endometrial carcinoma.
276
Conversely,
the use of metformin seems associated with an improvement in
overall survival for patients with endometrial carcinoma and a
reduced risk of cancer relapse.
277
In addition, metformin is associ-
ated with improvement in the overall survival of patients with endo-
metrial carcinoma with diabetes.
Recommendations
► Patients who are candidates for fertility- preserving treatment
must be referred to specialized centers. Fertility- sparing treat-
ment should be considered only in patients with AH/EIN or
grade 1 endometrioid endometrial carcinoma without myome-
trial invasion and without genetic risk factors (V, A).
► In these patients, endometrial biopsy, preferably through
hysteroscopy, must be performed (III, A).
► AH/EIN or grade 1 endometrioid endometrial carcinoma must
be conrmed/diagnosed by a pathologist experienced in gyne-
cological pathology (V, A).
► Radiologic imaging to assess the extension of the disease must
be performed. An expert ultrasound examination can substitute
pelvic MRI scan (III, B).
► Patients must be informed that fertility- sparing treatment is
not a standard treatment. Only patients who strongly desire
to preserve fertility should be treated conservatively. Patients
must be willing to accept close follow- up and be informed of
the need for future hysterectomy in case of failure of treatment
and/or after pregnancies (V, A).
Management and follow-up for fertility preservation
To date, there are no available randomized controlled trials
comparing different methods of conservative treatment in women
with AH/EIN or presumed stage IA grade 1 endometrioid carci-
noma. Existing data suggest that patients who received hystero-
scopic resection followed by progestin therapy achieve the highest
complete remission rate compared with other existing fertility-
preserving treatments.
263–269 278–295
Intrauterine progestin therapy
such as levonorgestrel- releasing intrauterine system combined with
gonadotropin- release hormone receptor agonist/progestin have a
satisfactory pregnancy rate and low recurrence rate. Patients who
received oral progestin only might be more likely to recur and have
more systemic adverse effects.
Recommendations
► All patients should be evaluated before and after the fertility-
sparing treatment at a fertility clinic (IV, C).
► Hysteroscopic resection prior to progestin therapy can be
considered (III, B).
► Medroxyprogesterone acetate (400–600 mg/day) or megestrol
acetate (160–320 mg/day) is the recommended treatment.
Treatment with levonorgestrel intrauterine device in combina-
tion with oral progestins with or without gonadotropin- releasing
hormone analogs can also be considered (IV, B).
► In order to assess response, hysteroscopic guided biopsy
and imaging at 3–4 and 6 months must be performed. If no
response is achieved after 6 months, standard surgical treat-
ment is recommended (IV, B).
► Continuous hormonal treatment should be considered in
responders who wish to delay pregnancy (IV, B).
► Strict surveillance is recommended every 6 months with TVUS
and physical examination. During follow- up, hysteroscopic
and endometrial biopsy should be performed only in case of
abnormal uterine bleeding or atypical ultrasound ndings (IV,
B).
► Fertility- sparing treatment can be considered for intrauterine
recurrences only in highly selected cases under strict surveil-
lance (IV, C).
► Hysterectomy and bilateral salpingo- oophorectomy is recom-
mended after childbearing due to a high recurrence rate. Pres-
ervation of the ovaries can be considered depending on age
and genetic risk factors (IV, B).
Synchronous presentation of low-grade endometrioid
endometrial and ovarian carcinomas
Adnexal involvement by endometrial carcinoma is currently a
parameter important in FIGO staging and has an impact on overall
survival rate.
296
It was shown that patients with simultaneous
involvement of the endometrium and ovary by low- grade endo-
metrioid carcinoma had a favorable outcome. This suggested that
they were synchronous primary tumors rather than metastatic
sites. Several criteria have been used in the past to distinguish
between endometrial carcinoma with ovarian metastasis and
synchronous primary tumors.
297 298
However, these were not easy
to apply.
Recent studies have shown that, for low- grade endometrioid
carcinomas, there is a clonal relationship between endometrial and
ovarian carcinomas in the vast majority of cases, indicating that
the carcinoma arises in the endometrium and extends secondarily
to the ovary.
299 300
In the most recent edition of WHO (2020) it is
mentioned that patients with clonally related low- grade endome-
trioid carcinomas should be managed without adjuvant treatment
(as if they were two independent primaries) when fullling the
following criteria: (1) low- grade endometrioid morphology, (2) no
more than supercial myometrial invasion, (3) absence of LVSI, and
(4) absence of additional metastases.
33 301
Recommendation
► If all WHO 2020 criteria mentioned above are met and the
ovarian carcinoma is pT1a, no adjuvant treatment is recom-
mended (III, B).
ADJUVANT TREATMENT
Adjuvant treatment recommendations for endometrial carcinoma
strongly depend on the prognostic risk group (see Table 2 for
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2020-002230 on 18 December 2020. Downloaded from

20
ConcinN, etal. Int J Gynecol Cancer 2021;31:12–39. doi:10.1136/ijgc-2020-002230
Joint statement
denitions of the prognostic risk groups with and without known
molecular classication).
Low risk
For patients with low- risk endometrial carcinoma, no adjuvant treat-
ment is recommended based on data from multiple randomized
trials.
302–305
For patients with stage I–II POLEmut endometrial carci-
nomas, no adjuvant treatment seems justiable based on the data
from independent series showing very few recurrences and also
in cases of observation.
21 25
For stage III patients, however, there
are only indirect data to support this, as all cases with advanced
disease had adjuvant treatment. In the molecular analysis of the
PORTEC-3 trial of high- risk endometrial carcinoma, those with
POLEmut endometrioid carcinoma had an excellent outcome in both
arms.
22
However, both trial arms included EBRT. Prospective regis-
tration (preferably in national or international studies) of POLEmut
endometrial carcinoma cases with treatment and outcome data is
strongly recommended.
Recommendations
► For patients with low- risk endometrial carcinoma, no adjuvant
treatment is recommended (I, A).
► When molecular classication is known:
– For patients with endometrial carcinoma stage I–II, low- risk
based on pathogenic POLE- mutation, omission of adjuvant
treatment should be considered (III, A).
– For the rare patients with endometrial carcinoma stage
III–IVA and pathogenic POLE-mutation, there are no out-
come data with the omission of the adjuvant treatment.
Prospective registration is recommended (IV, C).
Intermediate risk
Adjuvant brachytherapy provides excellent vaginal control and
high survival rates, similar to those after adjuvant EBRT in this
intermediate- risk population, as shown in large randomized trials,
particularly the PORTEC-2 trial and Swedish trial.
306–314
It was also
shown that only the small minority of patients with higher risk
based on substantial LVSI, p53abn, or L1CAM overexpression had a
slightly higher risk of pelvic recurrence with vaginal brachytherapy
than those who had EBRT. Therefore, the intermediate- risk category
only includes those with none or only focal LVSI and no p53abn. In
a Danish population study it was conrmed that the risk of locore-
gional relapse was higher (about 14%) with omission of vaginal
brachytherapy, but that overall survival was not different due to
treatment of relapse.
315
Therefore, no adjuvant treatment is an
option in this group, especially for patients aged <60 years who
have a lower risk of relapse.
MMRd and, especially, NSMP cancers form the majority of
endometrioid carcinomas and have an intermediate prognosis, in
between POLEmut (excellent prognosis) and p53abn carcinomas
(unfavorable prognosis). Findings of prior large randomized trials
in high–intermediate- risk endometrial carcinoma are therefore
mainly applicable to MMRd and NSMP endometrioid carcinomas in
this intermediate- risk category.
It has to be stressed that p53abn carcinomas restricted to
a polyp or without myometrial invasion were not included in the
randomized trials and the value of chemotherapy and of EBRT are
uncertain. Since the studies mentioned above did not include and/
or did not address non- endometrioid (and/or p53abn) carcinomas
without myometrial invasion, there are very few specic available
data on the best treatment for stage IA non- endometrioid carci-
nomas (serous, clear cell, undifferentiated carcinoma, carcinosar-
coma, mixed) without myometrial invasion. Some case series and
a recent analysis using the US National Cancer Data Base suggest
that adjuvant chemotherapy (with or without vaginal brachytherapy)
might improve survival, while other reports showed good outcomes
with vaginal brachytherapy only.
306
Therefore, these carcinomas
have been grouped in the intermediate- risk category and adjuvant
therapy should be discussed on a case- by- case basis until more
prospective data are available.
Recommendations
► Adjuvant brachytherapy can be recommended to decrease
vaginal recurrence (I, A).
► Omission of adjuvant brachytherapy can be considered (III, C),
especially for patients aged <60 years (II, A).
► When molecular classication is known, POLEmut and p53abn
with myometrial invasion have specic recommendations (see
respective recommendations for low- and high- risk).
► For p53abn carcinomas restricted to a polyp or without myome-
trial invasion, adjuvant therapy is generally not recommended
(III, C).
High–intermediate risk (pN0 after lymph node staging)
The denition of high–intermediate risk has changed in compar-
ison with the ESMO- ESGO- ESTRO consensus conference. In the
current prognostic risk group classication (see Table2), stage IA
endometrioid carcinomas are only included if there is substantial
LVSI.
3–5
This high–intermediate- risk group also includes stage IB
low- grade endometrioid with substantial LVSI, and stage IB high-
grade endometrioid carcinomas regardless of LVSI, and stage II
endometrioid carcinomas. In view of the higher risk of recurrence
in this newly classied group (even with negative nodes), adjuvant
brachytherapy can be recommended to decrease vaginal recur-
rence. In the case of substantial LVSI and/or stage II, EBRT can be
considered as it has been shown to reduce the risk of pelvic and
para- aortic nodal relapse.
316
In two older randomized controlled trials
317 318
there was no
difference between adjuvant chemotherapy alone and EBRT
alone in recurrence- free and overall survival. In the NSGO/EORTC
trial and the PORTEC-3 trials, the combination of chemotherapy
and radiotherapy seemed to provide better recurrence- free and
overall survival outcomes respectively compared with radiotherapy
alone.
319 320
The GOG-249 trial did not nd benet in recurrence-
free or overall survival from three cycles of chemotherapy with
brachytherapy compared with EBRT alone.
316
Molecular analysis
of PORTEC-3 trial tissues suggested no benet of chemotherapy
for MMRd carcinomas.
320 321
Omission of adjuvant treatment is an
option and this should be considered only when close follow- up
is guaranteed to ensure detection and prompt treatment of recur-
rence at an early stage.
Recommendations
► Adjuvant brachytherapy can be recommended to decrease
vaginal recurrence (II, B).
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2020-002230 on 18 December 2020. Downloaded from

21
ConcinN, etal. Int J Gynecol Cancer 2021;31:12–39. doi:10.1136/ijgc-2020-002230
Joint statement
► EBRT can be considered for substantial LVSI and for stage II
(I, B).
► Adjuvant chemotherapy can be considered, especially for high-
grade and/or substantial LVSI (II, C).
► Omission of any adjuvant treatment is an option (IV, C).
► When molecular classication is known, POLEmut and p53abn
have specic recommendations (see respective recommenda-
tions for low- and high- risk).
High–intermediate risk cN0/pNx (lymph node staging not
performed)
In view of the recent randomized trials GOG-249 (for stage I and II
endometrial carcinomas with high- risk factors or serous or clear
cell histology), the PORTEC-3 trial and the older GOG-99 trial, adju-
vant EBRT is recommended in case of substantial LVSI or stage
II.
302 316 319 320 322
Additional chemotherapy can be considered, espe-
cially for high- grade carcinomas, based on the PORTEC-3 trial, but
the question remains whether the benet outweighs the toxicity for
stage I–II endometrioid carcinomas, and multi- disciplinary shared
decision- making is needed.
320
Molecular analysis of PORTEC-3 trial
tissues suggested no benet of chemotherapy for MMRd carci-
nomas.
320 321
Adjuvant brachytherapy alone can be considered for
LVSI negative cases and for stage II grade 1 disease.
Recommendations
► Adjuvant EBRT is recommended, especially for substantial LVSI
and/or for stage II (I, A).
► Additional adjuvant chemotherapy can be considered, espe-
cially for high- grade and/or substantial LVSI (II, B).
► Adjuvant brachytherapy alone can be considered for high- grade
LVSI negative and for stage II grade 1 endometrioid carcinomas
(II, B).
► When molecular classication is known, POLEmut and p53abn
have specic recommendations (see respective recommenda-
tions for low- and high- risk).
High risk
The risk category changes also have a substantial impact on this
category. Some carcinomas designated as high risk in the ESMO-
ESGO- ESTRO consensus conference are not included anymore in
the high- risk sub- group in these ESGO- ESTRO- ESP guidelines.
3–5
High- risk carcinomas are now either stage III–IVA without residual
disease or stage I–IVA p53abn or non- endometrioid carcinomas
without residual disease with myometrial invasion (for specics see
Table2).
In 2019 the updated results of the PORTEC-3 trial, with a longer
median follow- up of 72 months and with 75% of participants
having reached 5 years of follow- up, were published.
323
In this
trial comparing combined chemotherapy and radiotherapy (two
cycles of cisplatin during radiotherapy followed by four cycles
of carboplatin- paclitaxel) with radiotherapy alone, a statistically
signicant 5% overall survival benet at 5 years and a 7% failure-
free survival benet was seen in the combined therapy group
compared with radiotherapy alone. The greatest overall survival
difference was seen in stage III carcinomas and in serous carci-
nomas regardless of stage. The GOG-258 trial compared the same
chemotherapy- radiotherapy schedule used in PORTEC-3 with six
cycles of carboplatin- paclitaxel chemotherapy alone and found
overlapping relapse- free and overall survival rates.
324
However, the
chemotherapy alone arm had signicantly higher rates of pelvic
and peri- aortic nodal relapse. Therefore, chemotherapy alone is
an alternative option based on the GOG-258 results for stage III–
IV disease. The nal analysis of the GOG-249 trial highlighted that
a post- operative adjuvant strategy of vaginal cuff brachytherapy
followed by three cycles of paclitaxel and carboplatin chemotherapy
did not signicantly increase 5- year recurrence- free survival or
5- year overall survival compared with pelvic radiotherapy.
325
Vaginal and distant recurrence rates were similar between arms.
However, pelvic or para- aortic nodal recurrences were signicantly
less common with pelvic radiotherapy. The older pooled analysis
of the NSGO- EORTC and MANGO- ILIADE trials used sequential
chemotherapy and radiotherapy (either sequence) and reported
signicantly longer recurrence- free survival compared with radio-
therapy alone.
319
Multiple retrospective studies indicated a survival
benet in patients with advanced stage endometrial carcinoma
treated with post- operative combined treatment including radio-
therapy and chemotherapy, delivered by either the sandwich or
sequential method, compared with radiotherapy alone or chemo-
therapy alone.
326–344
The benet of added chemotherapy is unclear for patients with
stage I–II clear cell carcinomas. These have often been included
with serous as 'non- endometrioid carcinomas'. Of note, in the
PORTEC-3 trial it was specically in those with serous histology
that a signicant benet of added chemotherapy was seen.
323
However, this was not observed in the NSGO- EORTC and MANGO-
ILEADE trials. Extended eld radiotherapy is used in the case of
involved para- aortic nodes or involvement of high common iliac
nodes, both with or without chemotherapy. The combination of
extended eld radiotherapy with chemotherapy using modern
intensity- modulated radiation therapy/volumetric modulated arc
therapy (IMRT/VMAT) techniques has been shown feasible in the
PORTEC-3 and GOG-258 trials. An additional brachytherapy boost
can be considered, especially for substantial LVSI, endocervical
stromal invasion, and/or stage IIIB–IIIC.
MMRd and NSMP carcinomas are included in the high- risk cate-
gory if stage III–IVA with no residual disease. The p53abn carci-
nomas can be of endometrioid, serous, undifferentiated, and clear
cell histologic type, but all consistently show a poor outcome and
should therefore be regarded as high risk. Based on the current
data, it is more difcult to draw conclusions regarding carcino-
sarcomas and undifferentiated carcinomas that are NSMP endo-
metrial carcinomas due to the lack of large series. For clear cell
carcinomas, the available data suggest some prognostic infor-
mation may lie in the molecular classication. About 40–50% of
clear cell carcinomas are p53abn. While serous carcinomas in the
PORTEC-3 trial had an unfavorable outcome and signicant benet
of added adjuvant chemotherapy, those with clear cell carcinomas
seemed to have an outcome similar to high- grade carcinomas in
general and were more favorable if not p53abn.
321 323
The ndings
of the randomized trials for endometrioid carcinomas cited above
are therefore largely applicable to stage III MMRd and NSMP carci-
nomas and to stage I–III p53abn carcinomas. This was also seen
in the molecular analysis of the PORTEC-3 trial, which showed a
statistically signicant survival advantage for p53abn carcinomas
with combined therapy for stage I–III. In contrast, POLEmut carci-
nomas had almost no recurrences in both arms. There was no clear
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2020-002230 on 18 December 2020. Downloaded from

22
ConcinN, etal. Int J Gynecol Cancer 2021;31:12–39. doi:10.1136/ijgc-2020-002230
Joint statement
benet of added chemotherapy for MMRd, while the NSMP carci-
nomas had some benet of added chemotherapy especially in case
of stage III. Prospective evaluation of the molecular characteristics
in randomized trials is highly recommended.
Recommendations
► EBRT with concurrent and adjuvant chemotherapy (I, A) or
alternatively sequential chemotherapy and radiotherapy is
recommended (I, B).
► Chemotherapy alone is an alternative option (I, B).
► Carcinosarcomas should be treated as high- risk carcinomas
(not as sarcomas) (IV, B).
► When the molecular classication is known, p53abn carci-
nomas without myometrial invasion and POLEmut have specic
recommendations (see respective recommendations for low-
and intermediate- risk) (III, C).
ADVANCED DISEASE
Surgery for clinically overt stage III and IV disease
In stage III and IV endometrial carcinoma (including carcinosarcoma),
maximal cytoreduction should be considered only if macroscopic
complete resection is feasible with acceptable morbidity.
345–350
Surgery should be performed in a specialized center. Pre- operative
complete staging and multi- disciplinary discussion within a tumor
board should be performed. Suspicious enlarged lymph nodes
should be resected if complete resection is possible.
351 352
A full
systematic pelvic and para- aortic lymphadenectomy of non-
suspicious lymph nodes should not be performed because there
is no evidence of a therapeutic impact. If upfront surgery is not
feasible or acceptable and therefore primary systemic therapy is
given, delayed surgery can be considered in case of a meaningful
response to chemotherapy.
353–360
Recommendations
► In stage III and IV endometrial carcinoma (including carcino-
sarcoma), surgical tumor debulking including enlarged lymph
nodes should be considered when complete macroscopic
resection is feasible with an acceptable morbidity and quality of
life prole, following full pre- operative staging and discussion
by a multi- disciplinary team (IV, B).
► Primary systemic therapy should be used if upfront surgery is
not feasible or acceptable (IV, A).
► In cases of a good response to systemic therapy, delayed
surgery can be considered (IV, C).
► Only enlarged lymph nodes should be resected. Systematic
lymphadenectomy is not recommended (IV, B).
Unresectable primary tumor due to local extent of disease
For patients presenting with unresectable locally advanced disease
and no evidence of multiple distant metastases, treatment options
include denitive radiotherapy or neoadjuvant chemotherapy
followed by surgery or denitive radiotherapy, depending on
response.
261 354–356 361
Denitive radiotherapy comprises EBRT to
the pelvis followed by image- guided brachytherapy. Concurrent
chemotherapy may be considered to enhance the radiation effect.
Brachytherapy should boost sites of macroscopic disease in the
uterus, parametrium, or vagina using the ESTRO principles. Adju-
vant chemotherapy should also be considered following primary
local treatment (surgery or radiotherapy) to reduce the risk of
distant metastases.
Recommendations
► For unresectable tumors, multi- disciplinary team discussion
should consider denitive radiotherapy with EBRT and intra-
uterine brachytherapy, or neoadjuvant chemotherapy prior
to surgical resection or denitive radiotherapy, depending on
response (IV, C).
► Image- guided brachytherapy is recommended to boost intrau-
terine, parametrial, or vaginal disease (IV, A).
► Chemotherapy should be considered after denitive radio-
therapy (IV, B).
Residual pelvic or para-aortic lymph nodes following surgery
Residual lymph node disease can be treated with EBRT using an
integrated or sequential boost to escalate the nodal dose. An IMRT
technique reduces the risk of toxicity to surrounding tissue.
362
Adjuvant chemotherapy reduces the risk of distant metastases for
patients with lymph node involvement.
320 323 324
Recommendations
► Residual lymph node disease should be treated with a combi-
nation of chemotherapy and EBRT (III, B) or chemotherapy
alone (IV, B).
► EBRT should be delivered to pelvis and para- aortic nodes
with dose escalation to involved nodes using an integrated or
sequential boost (IV, B).
Residual pelvic disease (positive resection margin, vaginal
disease, pelvic side wall disease)
Patients with residual pelvic disease following surgery have a high
risk of both local and distant recurrence. Radiotherapy can achieve
long- term local control while chemotherapy reduces the risk of
distant metastases. An individualized approach with either (chemo)-
radiotherapy to the pelvis followed by chemotherapy or adjuvant
chemotherapy followed by radiotherapy to the pelvis±para- aortic
nodes should be considered.
Recommendation
► An individualized approach with either radiotherapy or chemo-
therapy or a combination of both modalities should be consid-
ered by a multi- disciplinary team (V, B).
RECURRENT DISEASE
Radiotherapy naïve patients
Treatment of patients with recurrent endometrial carcinoma
involves a multi- disciplinary approach with surgery, radiotherapy,
and/or systemic therapy depending on the tness and wishes of
the patient, the tumor dissemination patterns, and prior treatment.
A decision about surgery needs to take account of patient morbidity
and wishes, available non- surgical treatments, and resources. The
interval between primary treatment and recurrence should also be
taken into consideration. Patients with recurrent disease, including
resectable peritoneal and lymph node relapse, should be consid-
ered for surgery only if it is anticipated that complete resection of
macroscopic disease can be achieved with a reasonable morbidity
prole.
363–369
The extent of the operation will depend on the degree
of tumor dissemination pattern.
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2020-002230 on 18 December 2020. Downloaded from

23
ConcinN, etal. Int J Gynecol Cancer 2021;31:12–39. doi:10.1136/ijgc-2020-002230
Joint statement
Locoregional recurrence of endometrial carcinoma is rare. With
the advent of modern image- guided radiation therapy, including
IMRT and image- guided adaptive brachytherapy, radiotherapy
has become the treatment of choice in previously non- irradiated
patients with isolated vaginal recurrence or locoregional recur-
rence.
363 364 370–379
Consideration should be given to remove solitary
easily accessible vaginal relapses, for better local symptom control
prior to radiotherapy.
Recommendations
► Patients with recurrent disease (including peritoneal and lymph
node relapse) should be considered for surgery only if it is
anticipated that complete removal of macroscopic disease can
be achieved with acceptable morbidity. Systemic and/or radia-
tion therapy should be considered post- operatively depending
on the extent and pattern of relapse and the amount of residual
disease (IV, C).
► In selected cases, palliative surgery can be performed to alle-
viate symptoms (eg, bleeding, stula, bowel obstruction) (IV, B).
► For locoregional recurrence, the preferred primary therapy
should be EBRT±chemotherapy with brachytherapy (IV, A).
► An easily accessable supercial vaginal tumor can be resected
vaginally prior to radiotherapy (IV, C).
► For vaginal cuff recurrence:
– Pelvic EBRT+intracavitary (±interstitial) image- guided
brachytherapy is recommended (IV, A).
– In case of supercial tumors, intracavitary brachytherapy
alone can be considered (IV, A).
► Systemic treatment can be considered before or after radio-
therapy (IV, C).
Radiotherapy pre-treated patients with locoregional
recurrence
In patients who have previously received EBRT±brachytherapy,
radical surgery with the intention of complete resection with clear
margins should be considered in specialized centers after ruling out
metastatic disease with modern imaging. Pelvic exenteration may
be considered for central local relapse.
349 380 381
Otherwise, further
radiation should be considered as radical therapy with or without
systemic therapy. Interstitial brachytherapy (low- dose rate or high-
dose rate) as the sole modality of treatment or combined with EBRT
can result in high local control over 1–5 years.
374
375 382 383
Other
techniques like permanent seed implant or post- operative elec-
tron irradiation, protons and stereotactic body radiotherapy may be
recommended in highly selected patients.
384–386
The appropriate
dose for each case needs to be individualized. Some low- dose rate
data suggest improved outcomes with doses >50 Gy. The high- dose
rate data are more varied, suggesting improved local control with
doses >40 Gy. In general, a longer time interval between the rst
and second course of radiation as well as recurrences <2–4 cm
tend to have improved outcomes. Multi- disciplinary management is
critical to develop individualized plans and to clearly communicate
potential side effects and expected treatment outcomes.
Recommendations
► In patients with a history of previous radiation, radical surgery,
including exenteration, should be considered when the inten-
tion is complete resection with clear margins (IV, B).
► Additional options to consider include intra- operative electron
radiation therapy or other forms of radiation therapy (IV, C).
► If surgery is not feasible, radical re- irradiation options include
stereotactic body radiotherapy targeting the recurrence,
permanent seed implants, or proton therapy. In selected cases,
limited volume re- irradiation with EBRT and brachytherapy
boost may be an option (especially if longer interval from the
rst irradiation) (IV, C).
► In patients who only had previous brachytherapy,
EBRT+brachytherapy boost is recommended (IV, C).
► In patients where re- irradiation with ERBT is not an option,
image- guided interstitial brachytherapy only is recommended
(may improve outcome) (IV, C).
Oligometastatic recurrent disease
Oligometastases is a disease concept that is dened by a state of
limited metastatic tumors for which local ablative therapy could be
curative. It refers in general to cancer patients with 1–5 metastases
or recurrences.
387–389
In recent years the concept of oligometastatic
relapse has evolved and has led to a change in the approach to
treatment. A prolonged disease- free interval and perhaps even cure
may be achieved in some situations where the primary cancer site
(if still present) is controlled and metastatic sites are ablated (surgi-
cally or with radiation).
390–393
Multi- disciplinary management is
critical to develop individualized plans and to communicate poten-
tial side effects and expected treatment outcomes. The additional
benet of chemotherapy is uncertain.
Recommendations
► Patients with oligometastatic disease should be considered for
radical local therapy (IV, B).
► Treatment options include (IV, B):
– Surgery
– Radiation therapy including stereotactic radiotherapy
– Local ablating techniques
► The additional benet of chemotherapy is uncertain (IV, B).
Systemic treatment for recurrent disease
Hormonal treatment results in a response rate of up to 55% in
advanced/recurrent endometrial carcinoma.
394
Low- grade, slowly
progressing, hormone receptor- positive tumors appear to gain
the greatest benet from treatment; however, clinical benet has
also been observed in patients with hormone receptor- negative
tumors.
395
Progestogens are generally recommended.
395
Alterna-
tive options include aromatases inhibitors, tamoxifen, and fulves-
trant. In the PARAGON trial a response rate of 7% and a clinical
benet rate of 44% was reported with anastrazole in a cohort of
82 patients with recurrent, receptor positive, endometrial carci-
noma.
396
A single- arm phase II trial demonstrated a high response
rate and clinical benet rate with the combination of letrozole and
everolimus.
397
Conrmation of hormone receptor status by biopsy
should be considered at the time of recurrence because of a poten-
tial change in hormone receptor expression between primary tumor
and recurrence. In patients undergoing hormonal therapy, the risk
of thrombo- embolic events needs to be taken into account. Proph-
ylaxis with low molecular weight heparin should be considered in
patients at high risk for thrombosis and be given according to local
guidelines. There are no universally agreed recommendations to
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2020-002230 on 18 December 2020. Downloaded from

24
ConcinN, etal. Int J Gynecol Cancer 2021;31:12–39. doi:10.1136/ijgc-2020-002230
Joint statement
predict a response to hormonal therapy in endometrial carcinoma
based on estrogen and progesterone receptor immunohistochem-
ical status. Some of the following should be taken into account:
(1) a wide range of hormonal agents are used, including medroxy-
progesterone acetate and synthetic progestational agents, lutein-
izing hormone releasing hormone antagonists, tamoxifen, and new
generations of selective estrogen receptor modulators; each has a
different molecular action and may therefore have different activity;
(2) receptor- negative status is not an absolute contra- indication to
hormone treatment; (3) in some reports, response rates to various
hormonal treatments for patients with endometrial carcinoma are
higher for those with progesterone receptor expression; (4) the
methodology for assessing and scoring hormone receptor expres-
sion in endometrial carcinoma is variable in the reported series;
(5) assessment of estrogen and progesterone receptor status in
the primary tumor may not reect the status in the recurrent or
metastatic tumor and thus a biopsy of recurrent or metastatic carci-
nomas for hormone receptor analysis may be helpful; (6) from a
pragmatic viewpoint, it seems reasonable to interpret a carcinoma
as receptor positive when immunoreactivity for estrogen receptor
or progesterone receptors is found in more than 1% of carcinoma
cells, until stronger validated scientic evidence is provided.
The combination of carboplatin and paclitaxel is the standard
chemotherapy treatment of advanced/recurrent endometrial carci-
noma based on a randomized phase 3 trial comparing carboplatin-
paclitaxel versus carboplatin- paclitaxel- anthracyclines that
reported overlapping progression- free survival and overall survival
between the two arms but an increased toxicity for the triple combi-
nation.
398
No standard treatment has been identied as second- line
therapy; a response rate of about 10–15% has been seen among
all the available treatment options. Thus, enrollment of patients in
clinical trials is strongly encouraged. Weekly paclitaxel and anthra-
cyclines (including pegylated liposomal doxorubicin when available)
are considered to be active drugs. The re- introduction of carbo-
platin may be considered after a prolonged interval from the last
platinum treatment, based on the results of a single- center retro-
spective series in patients treated with a median platinum- free
interval of 25 (8–79) months. A response rate of 50% and median
progression- free and median overall survival of 10 and 27 months,
respectively, was reported after platinum re- challenge.
399
Several anti PD-1 and anti PD- L1 checkpoint inhibitors have
been shown to have activity in endometrial carcinoma and thus far
pembrolizumab has been approved by the Food and Drug Admin-
istration (FDA) based on the results of a phase 2 single arm trial
for the treatment of MSI- high (MSI- H)/MMRd solid tumors that
have progressed on conventional therapy.
400 401
The combination of
intravenous pembrolizumab and lenvatinib, an oral multi- receptor
tyrosine kinase inhibitor, received FDA approval in October 2019
for the second- line systemic therapy of microsatellite- stable (ie,
non- MSI- H/MMRd) endometrial carcinoma based on the results
of a phase 2 single- arm trial reporting 36% response rate in this
population, including signicant activity in those with serous carci-
noma.
402 403
No phase 3 randomized data are yet available.
Approximately 30% of uterine serous carcinomas show HER2/
neu over- expression. A small randomized phase 2 trial of paclitaxel
and carboplatin with or without trastuzumab in HER2/neu positive
disease showed a 4.6 month increase in median progression- free
survival.
404
Anti- angiogenic agents and PI3kinase/mTor and MEK
inhibitors also have demonstrated activity but secure evidence of
benet is inconclusive due to the limited sample size of the trials,
inconsistency of results, and the low therapeutic index of the drugs,
suggesting further investigations in well- designed and properly
powered molecularly driven randomized trials are warranted.
405–416
Recommendations
► Hormone therapy is the preferred front- line systemic therapy
for patients with low- grade carcinomas without rapidly
progressive disease (II, A).
► Progestogens (medroxyprogesterone acetate 200 (–300) mg
and megestrol acetate 160 mg) are recommended (III, A).
► Alternative options for hormonal therapies include aromatases
inhibitors, tamoxifen, fulvestrant (III, C).
► The standard chemotherapy treatment is carboplatin AUC 5–6
+ paclitaxel 175 mg/m
2
every 21 days for six cycles (I, A).
► There is no standard of care for second- line chemotherapy.
Doxorubicin and paclitaxel are considered the most active ther-
apies (IV, C).
► In patients with a long platinum- free interval, re- introduction of
platinum can be considered (IV, C).
► Anti- PD1- based immune therapy with pembrolizumab could be
considered for second- line therapy of MSI/MMRd carcinomas.
The combination of pembrolizumab and the multi- tyrosine-
kinase inhibitor lenvatinib could be considered for second- line
treatment of microsatellite- stable carcinomas (III, B). However,
its use may be limited due to regulatory approvals or reim-
bursement in different countries. Clinical trial participation
should be offered to all patients with relapse disease (V, B).
Palliative radiotherapy
Historically, radiotherapy has been an efcient treatment to palliate
bleeding and pain from pelvic disease or systemic metastases. This
results in rapid pain relief and temporary cessation of bleeding in
the majority of patients.
417
Recommendations
► Radiotherapy is indicated for palliation of symptoms related to
pelvic or systemic disease (IV, A).
► Hypofractionated small volume EBRT can be used for treating
primary disease in patients not t for radical treatment (IV, B).
PRINCIPLES OF RADIOTHERAPY
The following sections present the general principles, the princi-
ples of adjuvant radiotherapy, of denitive treatment, and of radio-
therapy for recurrent disease.
258–261 307 362 372 377 418–423
General principles
State- of- art techniques and radiotherapy dose are chosen based on
clinical ndings, pathology, and patient factors including co- mor-
bidities. For complex treatments or rare cases, referral to a special-
ized center is recommended. Prospective assessment of toxicity is
recommended. Patients should have counseling on pelvic care and
general and sexual rehabilitation whenever appropriate.
Adjuvant radiotherapy
Radiotherapy should preferably commence within 6 (–8) weeks of
surgery or be scheduled in relation to chemotherapy.
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2020-002230 on 18 December 2020. Downloaded from

25
ConcinN, etal. Int J Gynecol Cancer 2021;31:12–39. doi:10.1136/ijgc-2020-002230
Joint statement
EBRT
IMRT/VMAT techniques are recommended because the more
conformal dose distribution increases normal tissue- sparing
compared with a four- eld conventional or 3D- conformal plan.
424
The clinical target volume (CTV) includes the pelvic nodes (external
iliac, internal iliac, obturator, distal common iliac), parametria, and
upper vagina. The upper common iliac and sub- aortic pre- sacral
lymph nodes are included when there is cervical stromal involve-
ment and/or pelvic lymph node involvement. The lymph node
target volume may be extended to include the aortic bifurcation or
para- aortic nodes, up to or just above the level of the renal vessels,
depending on the location and number of positive lymph nodes, site
of sentinel lymph nodes, and whether there is extrauterine primary
tumor involvement. The CTV should be individualized when there
is a positive resection margin, pelvic peritoneal disease, or vaginal
involvement. Treatment with a comfortably full bladder reduces
the volume of irradiated small bowel and bladder. The planning
target volume (PTV) should account for potential internal motion,
depending on the method of verication used during the course
of treatment. Image- guided radiotherapy by repeated volumetric
imaging with cone beam CT (and use of so- called library of plans
or plan of the day techniques) may enable the use of smaller CTV-
PTV margins to reduce normal tissue toxicity. The prescription
dose is commonly 45–50.4 Gy in 25–28 fractions over 5–6 weeks.
An integrated or sequential EBRT boost is given to residual lymph
node disease, sites of extracapsular nodal spread, and positive
lateral resection margins with a total dose of 55–60 Gy EQD2
10
for microscopic residual disease, or up to 66 Gy for macroscopic/
bulky disease. Concurrent and adjuvant chemotherapy may be
considered for stage III disease, serous histology and/or recurrent
disease.
Vaginal brachytherapy
Vaginal examination is undertaken to ensure the vaginal cuff is
healed and to assess the size and shape of the vagina to guide
applicator selection. Usually a vaginal cylinder is used but other
applicators can be used, depending on patient anatomy. The target
volume is individually determined and is usually the upper third of
the vagina to a depth of 5 mm (both superiorly and halfway along
the active length). The high- dose rate brachytherapy dose is most
commonly 21–24 Gy in 3–4 fractions to 0.5 cm from the applicator
surface, or 8–11 Gy in 2–3 fractions when given as a boost following
EBRT. A higher dose is required for treatment of residual disease
or positive margins. Pulsed- dose rate brachytherapy can be used
following EBRT to boost macroscopic residual disease witha dose
of 15–25 Gy. The treatment planning options are to use a standard
library plan for each applicator size and treatment length or to use
image- guided adaptive brachytherapy. In institutions where image-
guided adaptive brachytherapy is applied, imaging of the applicator
with CT scan or MRI evaluates whether the applicator is in close
apposition to the vaginal mucosa and close to organs at risk. This
allows verication and calculation of cumulative doses, especially if
vaginal brachytherapy is used as a boost after EBRT. Image- guided
adaptive brachytherapy is strongly recommended when there is
residual vaginal disease following surgery using similar principles
to treatment for recurrent disease.
Denitive treatment
Denitive radiotherapy with EBRT, brachytherapy, or a combination
of both is indicated for primary tumors where surgery is contra-
indicated for medical reasons. If patients are medically unt for
surgery, consider whether a long course of EBRT would be toler-
ated or, if not, a more hypofractionated approach could be used.
Intrauterine brachytherapy as a sole treatment modality is used for
low- grade early stage disease whereas the combination of EBRT
and intra- cavitary brachytherapy is recommended for high- grade
tumors and/or deep myometrial invasion. Specialist anesthetic
review may be required to assess suitability for brachytherapy or
whether brachytherapy could be applied with local anesthesia only.
More advanced inoperable disease is treated with a combination of
pelvic EBRT and intrauterine brachytherapy with or without concur-
rent platinum- based chemotherapy. EBRT is planned with at least
three- dimensional (3D) conformal radiotherapy to ensure inclusion
of the whole uterus. The preferred technique is intensity- modulated
radiotherapy with adaptive image guidance to verify target volume
coverage and to maximize normal tissue sparing. A highly conformal
EBRT boost (with IMRT or stereotactic body radiotherapy) can be
used to escalate the total dose to the tumor site in the uterus to at
least 65 Gy if brachytherapy is not feasible.
Image- guided adaptive brachytherapy is recommended, prefer-
ably using MRI at the time of brachytherapy, in order to optimize
tumor coverage and organ at risk doses. The brachytherapy appli-
cator should consist of an intrauterine applicator (preferably a
dedicated applicator with multiple channels for the larger uterus)
and a vaginal component depending on the extent of any extra-
uterine disease. Interstitial applications may be required to achieve
adequate coverage. In view of the rarity of denitive treatment for
endometrial carcinoma, referral to a dedicated center is recom-
mended. The tumor- related target volumes include the (residual)
gross tumor volume on MRI (GTV- res) and the CTV is the whole
uterus and any extrauterine sites of extension before EBRT. The
treatment plan aims include a total dose (EQD2
10
) of at least 80 Gy
to GTV- res, CTV D90 of about 48 Gy with brachytherapy alone, and
60–65 Gy with the combination of EBRT and brachytherapy.
Recurrent disease
Radiotherapy treatment for recurrent endometrial carcinoma
depends on the site of disease and any previous treatment. It
involves EBRT, brachytherapy, or a combination of both modalities.
Concurrent or sequential chemotherapy may also be considered.
Radiation-naïve or previous brachytherapy only
Pelvic EBRT is used according to the guidelines above. Brachytherapy
is used to boost recurrent disease in the vagina; in selected cases
with supercial tumors brachytherapy alone can be considered. The
brachytherapy applicator options include a vaginal cylinder or mold
for supercial lesions whereas interstitial applicators can be used
for bulkier tumors.
Image- guided adaptive brachytherapy is recommended, prefer-
ably using MRI at the time of brachytherapy, in order to optimize
tumor coverage and organ at risk doses. When image- guided adap-
tive brachytherapy is used, the target volumes should be contoured
according to the recent GEC- ESTRO recommendation for primary
vaginal cancer, aiming for a total dose (EQD2
10
) of 80–85 Gy
to CTV D90 with the combination of EBRT and image- guided
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2020-002230 on 18 December 2020. Downloaded from

26
ConcinN, etal. Int J Gynecol Cancer 2021;31:12–39. doi:10.1136/ijgc-2020-002230
Joint statement
brachytherapy.
422
If brachytherapy is not feasible due to tumor
location or topography, a sequential EBRT boost with conformal
radiotherapy, IMRT, or stereotactic body radiotherapy is used to
deliver a total GTV dose of at least 65 Gy EQD2
10
.
Re-irradiation
Re- irradiation is individualized according to the extent of disease,
previous radiation elds, and time elapsed from the previous treat-
ment. In general, recurrences with a longer disease- free interval
as well as recurrences less than 2–4 cm tend to have improved
outcomes. Ideally, this should be done in specialist centers with
prospective collection of dosimetric and clinical data. The most
common re- irradiation technique is intracavitary- interstitial
brachytherapy, preferably image- guided with CT scan or MRI.
421
However, in selected cases EBRT, stereotactic body radiotherapy,
proton or carbon ion therapy is an option, particularly for pelvic side-
wall or lymph node disease. Organ at risk dose constraints should
take into account prior radiotherapy treatment to derive cumulative
doses. Some low- dose rate data suggest improved outcomes with
doses more than 50 Gy. The high- dose rate data are more varied
with some studies suggesting improved local control with doses
more than 40 Gy EQD2
10
.
PRINCIPLES OF PATHOLOGIC EVALUATION
The following sections present the requirements for specimens
submitted for pathologic evaluation including specimen grossing
and sampling, for the pathology report, and the molecular classi-
cation.
19 21 23 26 425 426
The sections are proposed in agreement with
the recently published recommendations from the ISGyP and Inter-
national Collaboration on Cancer Reporting, and WHO Classication
of Tumors (5th edition).
9 33 427–429
Requirements for specimens submitted for pathologic
evaluation
Patient information, previous cytology, histologic specimens, clinical
and radiological data need to be included on the specimen request
form, particularly if there is no electronic patient le. This needs to
provide itemised details of biopsy and surgical specimen (type of
hysterectomy, presence of ovaries and fallopian tubes, presence of
lymph nodes, and designation of lymph node sites). Biopsies should
be sent to the pathology department in a container with liquid xa-
tive (10% neutral formalin is preferred). Surgical specimens should
be either sent in a xative or preferably fresh if there is a specic
workow for it and if the microbiological risk is controlled. This
allows proper opening of the uterus and sampling a fresh tissue for
research purposes.
Specimen grossing and sampling
All pathology reports should include a detailed section, code/block
key on which the origin/designation of all tissue blocks should be
recorded.
The specimen needs to be oriented, which means that the ante-
rior and posterior walls of the uterus are identied using anatomic
landmarks such as the peritoneal reection and the round ligament/
ovaries. All organs/structures received should be documented and
their measurements and gross appearance recorded.
The uterus should be opened immediately on receipt in the
pathology laboratory and placed in formalin within an hour of
opening whenever possible. If the uterus is not immediately sent
to a pathology laboratory, the uterine cavity needs to be opened
technically correctly to guarantee proper xation. The uterus is
preferably opened along the lateral uterine walls (3 and 9 o’clock),
although 12 and 6 o’clock sectioning may be acceptable.
The pathology laboratory personnel and/or pathologists should
manage the requests for fresh tissue for banking and/or investiga-
tional protocols and this task should be completed as soon as the
specimen is received in the pathology laboratory.
Inking of peritoneal and/or non- peritoneal surfaces is recom-
mended in hysterectomy specimens and is mandatory in radical
hysterectomy specimens in which the parametrium and vaginal
cuff are present.
At least the largest dimension of the tumor must be provided,
although providing three dimensions is recommended. Horizontal/
transverse sectioning is recommended. Sampling one section per
centimeter of the largest tumor dimension is recommended.
In case of pre- operative endometrial sampling with a malignant
diagnosis and no visible lesion on gross examination or a history
of atypical endometrial hyperplasia/EIN, the entire endometrium
and adjacent inner myometrium should be submitted for micro-
scopic examination. The same applies to hysterectomy specimens
that have been obtained for other reasons (leiomyomas, adeno-
myosis, etc) when the endometrium is grossly inconspicuous but
endometrial carcinoma or atypical endometrial hyperplasia/EIN are
detected on the initial histological sections.
At least one full- thickness section of the uterine wall including
serosa is required to show the deepest point of myometrial invasion.
The number of sections submitted should not be altered in the
context of adenomyosis. However, in cases where the assess-
ment of myometrial invasion is difcult because of tumor involving
adenomyosis, taking additional sections of the uterine wall may be
useful.
Whenever possible, the interface between the tumor and its
surroundings should be submitted for microscopic examination.
This facilitates the measurement of the depth of myometrial inva-
sion and the identication of precursor lesions.
At least one representative section of non- neoplastic endome-
trium should be submitted for microscopic examination. In addition,
any grossly identied endometrial lesions separate from the tumor
should be submitted.
All gross endometrial abnormalities need to be submitted for
microscopic examination in the hysterectomy specimen from
patients with Lynch syndrome. In the absence of a gross lesion,
the endometrium should be submitted in toto, including the lower
uterine segment.
A minimum of two sections (one anterior, one posterior) should
be submitted from the lower uterine segment.
Parametrial tissue/parametrium should be sampled before
opening the uterus as this approach minimizes the chance of
nding carryovers. All of the parametrial tissue/parametrium should
be submitted for histologic examination. If macroscopic tumor is
seen in the parametrial tissue/parametrium, the most proximal
parametrial section should include the adjacent outer portion of the
cervical wall.
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2020-002230 on 18 December 2020. Downloaded from

27
ConcinN, etal. Int J Gynecol Cancer 2021;31:12–39. doi:10.1136/ijgc-2020-002230
Joint statement
The cervix should be left attached to the corpus during the gross
examination of a hysterectomy specimen obtained for endometrial
carcinoma. At least two full thickness sections (one anterior and
one posterior) should be submitted from a grossly unremarkable
cervix. At least two representative sections of tumor involving the
cervix should be submitted when the cervix is grossly involved by
endometrial carcinoma. These sections must include the full thick-
ness of the cervical wall and the ectocervical or vaginal cuff margin.
Gross examination of a morcellated hysterectomy specimen
requires special attention to identify any endometrial abnormality,
although this may be extremely difcult to see in some cases. If
such an abnormality is detected, the entire endometrial lesion
and the adjacent myometrium should be submitted for micro-
scopic examination. In addition, sampling of myometrial tissue
containing any serosal surface should be undertaken. If the endo-
metrium appears grossly unremarkable and the initial represen-
tative sections demonstrate the presence of atypical endometrial
hyperplasia/EIN or endometrial carcinoma, careful re- grossing is
required with the submission of all the visible endometrial lining
and adjacent myometrium. If the morcellated specimen contains
the uterine cervix, this should be sampled representatively.
Gross examination of the fallopian tube must be carefully under-
taken and any areas with macroscopic abnormalities should be
submitted for microscopic examination. If the fallopian tube is
unremarkable, the entire tube should be submitted for microscopic
examination using the sectioning and extensively examining the
mbriated end (according to the SEE- FIM protocol), particularly for
serous carcinoma and carcinosarcoma, while only the mbrial end
should be submitted in toto in other scenarios using the guidelines
of the SEE- FIM protocol, along representative cross- sections of the
remainder of the fallopian tube.
Gross examination of the ovary must be carefully performed. In
case of endometrial serous, clear cell carcinoma or carcinosar-
coma, the entire ovary should be submitted after slicing it perpen-
dicularly to its long axis at 2–3 mm intervals. If possible, the same
protocol should be used for oophorectomy specimens accompa-
nying hysterectomies for other endometrial carcinoma histotypes.
Should the latter not be possible, at least two sections of each ovary
should be submitted.
Omentectomy is part of the staging procedure of endometrial
serous carcinoma, undifferentiated carcinoma, and carcinosar-
coma. The gross appearance and measurement of the omentum
should be provided. Omental tissue should be sliced at 0.5 cm
intervals to detect small abnormalities. If the omentum is grossly
positive, one or two representative sections are enough for micro-
scopic evaluation, but if it is grossly negative, one representative
section per 2 or 3 cm of maximal omental dimension or at least a
total of four blocks of tissue should be submitted.
Lymph nodes from different anatomic sites should be sent in
separate appropriately labeled specimen containers and handled
separately. They should be carefully dissected from the adipose
tissue. This can be done with a thorough visual examination and
palpation. A small amount of adipose tissue should be left around
larger lymph nodes to evaluate the presence or absence of extra-
nodal extension. Lymph nodes up to 2 mm are totally embedded. If
larger than 2 mm, parallel slices at 2–3 mm intervals perpendicular
to the long axis of the node should be performed. All grossly unre-
markable lymph node tissue should be submitted for microscopic
examination. The number of lymph nodes submitted per cassette
and the way they have been submitted—for example, in toto if
very small or sectioned—should be specied in the section code.
With grossly positive lymph nodes, representative sections to
demonstrate the largest size of tumor involvement as well as the
surrounding adipose tissue should be submitted for microscopic
examination and noted in the section code.
The description of the sentinel lymph node should include gross
measurement and description of gross appearance including the
presence of dye. The lymph node is sliced at 2–3 mm intervals
perpendicular to its long axis. A small rim of adipose tissue should
be left around the lymph node. The entire lymph node is submitted
for microscopic examination in properly coded cassettes. Ultrast-
aging is encouraged (ie, additional recuts and/or IHC for keratin). At
the present time there is no universal ultrastaging protocol.
Frozen section for intra- operative assessment is not encouraged
for myometrial invasion assessment because of poor reproducibility
and because it interferes with pre- analytical issues and the possi-
bility of carryovers.
Report of pathology results (required items)
► Description of the specimen(s) submitted for histologic
evaluation
► Attached anatomic structures
► Accompanying specimens
► Tumor type (WHO Classication of Tumors (5th edition))
► Tumor grade (FIGO and WHO Classication of Tumors (5th
edition)). Endometrioid endometrial carcinoma is graded using
FIGO grading criteria: grades 1, 2, and 3 tumors exhibit ≤5%,
6–50%, and >50% solid non- glandular (including cribriform),
non- squamous growth. The presence of severe cytologic
atypia in the majority of cells (>50%) increases the grade by
one level, but serous carcinoma should be excluded in cases
with nuclear atypia that is out of proportion to the architecture.
Binary grading is recommended by the WHO Classication of
Tumors (5th Edition) whereby grades 1–2 tumors are classied
as low- grade and grade 3 tumors as high- grade.
► Absence or presence and depth of myometrial invasion should
be reported in all endometrial carcinoma as 'none or less than
half' OR 'half or more'. The measurement should be performed
from the adjacent endometrial–myometrial interface.
► If myometrial invasion occurs from carcinoma within adeno-
myosis, the deepest myoinvasive point should be reported
according to where this is located in the myometrium, and
regardless of whether or not it arises from adenomyosis. In
case of an exophytic tumor, the depth of myometrial invasion,
and not tumor thickness, should be measured by identifying the
adjacent endo–myometrial junction and by correlating with the
macroscopic appearance. For tumors involving polyps, meas-
urement of invasion is performed only if the tumor invades the
underlying myometrium.
► LVSI should be unequivocal and reported as focal and exten-
sive/substantial (ve vessels or more). LVSI should not be
included in assessment of myometrial invasion depth.
► Cervical stromal invasion: for the purposes of standard
reporting, the uppermost endocervical mucinous gland iden-
tied in the section should be taken as the upper limit of the
endocervix.
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2020-002230 on 18 December 2020. Downloaded from

28
ConcinN, etal. Int J Gynecol Cancer 2021;31:12–39. doi:10.1136/ijgc-2020-002230
Joint statement
► Vaginal involvement.
► Uterine serosal involvement. Tumor inltrating the full myome-
trial thickness and reaching sub- mesothelial bro- connective
tissue or the mesothelial layer should be reported as serosal
involvement; tumor may or may not be present on the surface
of the uterus; a desmoplastic response may or may not be
present.
► Parametrial involvement.
► Adnexal involvement. Care should be taken to determine
whether the ovarian involvement is considered to be meta-
static or 'synchronous'. Synchronous low- grade endometrioid
carcinomas of the endometrium and the ovary have been
demonstrated mostly to be clonally related in the vast majority
of cases. Their reported indolent behavior supports conserva-
tive management when the following criteria are met: (a) both
tumors are low grade; (b) <50% myometrial invasion; (c) no
involvement of any other site; (d) absence of extensive LVSI
at any location. These parameters should be reported and
included in a specic comment.
In cases of serous endometrial carcinoma with co- existing
tubal intra- epithelial (mucosal) carcinoma, with or without
stromal invasion, ancillary techniques should be undertaken
to help dene whether the Fallopian lesion is independent or
metastatic. In cases of endometrioid endometrial carcinoma, a
comment may be included on the unknown prognostic signi-
cance of this nding.
► Omental involvement.
► Peritoneal involvement.
► Lymph node status including sentinel lymph node status
reports the total number of nodes found and the number of
positive lymph nodes, and the presence of extranodal extension
(list for all separates sites). Micrometastasis (>0.2 mm and up
to 2 mm) are reported as pN1(mi). ITCs no greater than 0.2 mm
in regional nodes should be reported as pN0 (i+).
► Pathologically proven distant metastases.
► Required ancillary techniques (IHC for p53, MSH-6 and PMS-2,
complemented with MLH-1 and MSH-2, MLH-1 promoter
methylation analysis in cases of MLH-1/PMS-2 decrease
expression). Additional immunohistochemical markers may be
important for pathologic diagnosis (PTEN, p16, ER, Napsin A,
Racemase, Pax8, E- Cadherin) or prognosis (L1CAM).
► Provisional pathologic staging pre- tumor board/multi-
disciplinary team meeting. The TNM staging system (Union for
International Cancer Control and American Joint Committee
on Cancer versions) for endometrioid carcinoma is largely
concordant with the widely used FIGO system.
Report of pathology results (recommended items unrelated to
stage and with limited supporting evidence)
► Tumor site.
► Tumor size.
► Percentages of different components of mixed carcinoma and
in carcinosarcoma.
► Measurement of absolute depth of myometrial invasion,
percentage of myometrium inltrated by tumor, invasion of
inner, middle, or outer one third of the myometrium, distance of
myo- invasive tumor to serosal surface.
► Microcystic, elongated, fragmented pattern of invasion.
► Peritoneal cytology (if available).
Recommended ancillary investigations.
Molecular classication
The decision to use molecular classication in all endometrial
carcinoma cases in the subset of high- grade or high- risk tumors
or in none of the cases depends on the availability of resources
and decision by the multi- disciplinary team of each center.
Molecular classication is recommended to be performed
by the TCGA surrogate using the diagnostic algorithm provided
by Vermij et al.
24
This diagnostic algorithm requires testing of
three immunohistochemical markers (p53, MSH-6, PMS-2) and
somatic mutation analysis of POLE (exons 9, 11, 13, 14). Guid-
ance on the interpretation of pathogenicity of POLE variants is
provided by Leon- Castillo et al.
26
Five categories of tumors are recognized: (1) ultramutated/with
pathogenic POLE mutations; (2) hypermutated with MSI/MMRd
(loss of MMR protein immunoreactivity); (3) high copy number/
p53abn (p53 mutant immunoreactive pattern); (4) low copy
number/NSMP (retained MMR protein immunoreactivity, and p53
wild- type immunoreactive pattern); (5) multiple classier (any
combination of markers included in the previous categories).
If available, molecular classication data should be integrated into
conventional pathologic diagnosis. The report should include informa-
tion regarding the methods used for IHC as well as for POLE mutation
analysis. It should include information from the literature regarding the
pathogenicity of each POLE mutation detected.
26
PSYCHO-ONCOLOGICAL SUPPORT FOR WOMEN WITH
ENDOMETRIAL CARCINOMA
Endometrial carcinoma, even as a cancer with a relatively good prog-
nosis, is a life- threatening disease. Treatment may produce signicant
toxicities which cause substantial short- and long- term side effects,
functional loss in various behavioral and life domains as well as psycho-
social distress. The patient and her caregivers may face major chal-
lenges in terms of coping and adjustment.
Therefore, continuous evaluation for psychological distress, sexual
dysfunction, and psychiatric co- morbidity as well as identication of
psychosocial needs are of major importance.
430
The rst step includes
an early assessment and identication of the patient’s distress.
431
There
are several standardized and validated screening instruments available
such as the Hospital Anxiety and Depression Scale or the easy to use
Distress Thermometer.
432
Depending on the result of the diagnostic
process, various interventions should be offered such as counseling,
individual or group psychotherapy, psychoeducational interventions,
art therapies, or relaxation techniques. For patients with a disease
involving genital organs, cancer itself, surgical treatment and subse-
quent hormonal loss may impair sexual function. Therefore, discussion
and treatment of sexual problems should be integrated as part of a
holistic approach.
In order to empower patients to cope with physical and
psychosocial long- term side effects of disease, treatment, and to
preserve quality of life, they should receive a personalized survi-
vorship care plan including information and education life style
and prevention of secondary malignancies and other diseases.
Contact with advocacy groups should be offered to all patients.
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2020-002230 on 18 December 2020. Downloaded from

29
ConcinN, etal. Int J Gynecol Cancer 2021;31:12–39. doi:10.1136/ijgc-2020-002230
Joint statement
Author afliations
1
Department of Gynecology and Obstetrics, Innsbruck Medical University,
Innsbruck, Austria
2
Evangelische Kliniken Essen- Mitte, Essen, Germany
3
Department of Pathology, Hospital Universitari Arnau de Vilanova, University of
Lleida, CIBERONC, Irblleida, Spain
4
Department of Pathology, Hospital Universitari de Bellvitge, University of
Barcelona, Idibell, Spain
5
Department of Gynecology and Obstetrics, Gynecologic Oncology, Leuven Cancer
Institute, Catholic University Leuven, Leuven, Belgium
6
Department of Obstetrics and Gynecology, First Faculty of Medicine, Charles
University, General University Hospital in Prague, Prague, Czech Republic
7
Department of Oncology, Rigshospitalet, Copenhagen University Hospital,
Copenhagen, Denmark
8
Department of Radiation Oncology, Medical Faculty of the University of Cologne,
Cologne, Germany
9
UCL Cancer Institute, University College, London, UK
10
Department of Pathology, Leids Universitair Medisch Centrum, Leiden,
Netherlands
11
Department of Radiation Oncology, Institut Gustave Roussy, Villejuif, France
12
Division of Gynecologic Oncology, Fondazione Policlinico Universitario A Gemelli
IRCCS, Rome, Italy
13
Department of Gynaecologic Oncology, Imperial College London Faculty of
Medicine, London, UK
14
Department of Medical Oncology, Clinica Universidad de Navarra, Madrid, Spain
15
Department of Pathology, Hospital Graz II, Graz, Austria
16
School of Medicine, Johannes Kepler University Linz, Linz, Austria
17
Department of Obstetrics and Gynecology, Innsbruck Medical University,
Innsbruck, Austria
18
Department of Surgery, Institut Gustave Roussy, Villejuif, France
19
Department of Radiotherapy, Erasmus MC Cancer Institute, Rotterdam,
Netherlands
20
Department of Medical Oncology, St James Hospital, Dublin, Ireland
21
Department of Obstetrics and Gynecologic Oncology, University Hospital,
Strasbourg, France
22
Histopathology and Molecular Diagnostics, Azienda Ospedaliero Universitaria
Careggi, Florence, Italy
23
Department of Gynecology with Center for Oncological Surgery, Campus
Virchow Klinikum, Charité–Universitätsmedizin Berlin, Corporate Member of Freie
Universität Berlin, Humboldt- Universität zu Berlin and Berlin Institute of Health,
Berlin, Germany
24
Department of Radiation Oncology, Comprehensive Cancer Center, Christian
Doppler Laboratory for Medical Radiation Research for Radiation Oncology, Medical
University of Vienna, Vienna, Austria
25
Department of Gynaecology, Royal Marsden Hospital, London, UK
26
Department of Medical Oncology, Amsterdam University Medical Centres,
Amsterdam, Noord- Holland, Netherlands
27
Department of Gynecology and Obstetrics, TU Dresden Medizinische Fakultat Carl
Gustav Carus, Dresden, Germany
28
Gynecologic Oncology Program, European Institute of Oncology, IRCCS, Milan and
University of Milan- Bicocca, Milan, Italy
29
Clinical Research Unit, Institut Bergonie, Bordeaux, France
30
Department of Radiation Oncology, Leiden University Medical Center, Leiden,
Netherlands
Presented at
These guidelines statements were developed by ESGO, ESTRO and ESP and are
published in the International Journal of Gynaecological Cancer, Radiotherapy &
Oncology and the Virchows Archiv.
Acknowledgements The authors thank ESGO, ESTRO, and ESP for their
support. ESGO ofce, especially Kamila Macku, provided invaluable logistical
and administrative support throughout the process. The authors also thank the
191 international reviewers (physicians and patient representatives, Appendix 2)
for their valuable comments and suggestions. The European Society for Medical
Oncology, Professor Cristiana Sessa and the ESMO- ESGO- ESTRO consensus
conference working group are gratefully acknowledged for the previous 2014
Endometrial Consensus Conference. The authors wish to express sincere gratitude
to Annette Hasenburg and Joachim Weis for describing the psycho- oncological
aspects in this article.
Contributors The development group (including all authors) is collectively
responsible for the decision to submit for publication. NCon (chair), CLC (co-
chair), XM- G (co- chair) and FP (methodologist) have written the rst draft of the
manuscript. All other contributors have actively given personal input, reviewed the
manuscript, and have given nal approval before submission.
Funding All costs relating to the development process were covered from ESGO,
ESTRO, and ESP funds.
Competing interests NCon: advisory boards for Seattle Genetics, AstraZeneca
and Mersana, education fees from Medscape Oncology, and grants for travelling
from Roche, Genmab and Amgen. IV: advisory boards for Amgen, AstraZeneca,
Clovis Oncology, Carrick Therapeutics, Debiopharm International, F Hoffmann- La
Roche, Genmab, GSK, Immunogen, Millenium Pharmaceuticals, MSD Belgium,
Octimet Oncology, Oncoinvent, Pharmamar- Doctaforum Servicios, Roche,
Sotio, Tesaro, Deciphera Pharmaceuticals and Verastem Oncology (fees for
consulting to his university), contracted research (KU Leuven) for Oncoinvent AS
and Genmab, corporate sponsored research for Amgen and Roche, and grants
for travelling from Amgen, MSD/Merck, Roche, AstraZeneca and Tesaro. DC:
advisory boards for AstraZeneca, Roche, Sotio and Novocure. MRM: personal
nancial interests for AstraZeneca, Biocard, Clovis Oncology, Geneos, Genmab,
Karyopharm Therapeutics, Merck, Mersana, MSD, Oncology Venture, Pzer, Roche,
SeatleGenetics, SeraPrognostics, Sotio, Tesaro- GSK, ZaiLab; leadership role for
Karyopharm Therapeutics, Sera Prognostics; institutional nancial interests (study
grants) for AstraZeneca, Boehringer Ingelheim, Clovis Oncology, Pzer, Tesaro-
GSK, Ultimovacs. JL: advisory boards for AstraZeneca, Pzer, GSK, Eisai, MSD/
Merck, Artios Pharma, Regeneron, Amgen and Clovis Oncology, and grants for
travelling from Clovis Oncology. CC: advisory boards for MSD, Takeda and GSK,
conducting research for TherAguiX and Roche, and grants for travelling from
Takeda. AF: advisory boards for GSK and Johnson & Johnson SpA, and grants for
travelling from Pharmmar and MSD Italia. CF: advisory boards for AstraZeneca,
Clovis, Ethicon, Roche, MSD, GSK and Tesaro, and grants for travelling from
Sequana. AGM: speakers’ bureau activities for AstraZeneca, Pharmamar, Roche
and GSK, advisory boards for Amgen, AstraZeneca, Clovis Oncology, Genmab,
GSK, Immunogen, Merck Sharp & Dohme, Novartis, Oncoinvent, Pzer/Merck,
Pharmamar, Roche and Sotio, and grants for travelling from AstraZeneca,
Pharmamar Roche and Tesaro. DL: advisory boards for Roche, Amgen, MSD, GSK,
Clovis, AstraZeneca, Immunogen, Genmab, Pharmamar and Merck, and grants
for travelling from Pharmamar, GSK, Roche and AstraZeneca. CM: consulting/
advisory boards for Roche, Novartis, Amgen, MSD, AstraZeneca, Pzer, Pharmamar,
Cerulean, Vertex and Tesaro, funded research from EU, FWF, AstraZeneca and
Roche, and honoraria/expenses from Roche, Novartis, Amgen, MSD, Pharmamar,
AstraZeneca and Tesaro. JS: advisory boards for Roche, Eisei, MSD, AstraZeneca,
Clovis, GSK and Tesaro. AT: advisory boards for Genmab; PW: advisory boards
for Amgen, AstraZeneca, MSD, Novartis, Pzer, Pharmamar, Lilly, Roche Pharma
GmbH, TEVA, Eisai, Clovis and Tesaro, and grants for travelling from Roche Pharma
GmbH, AstraZeneca, MSD, Amgen and Pzer. NC: consulting and advisory services,
speaking or writing engagements, public presentations for Roche, AstraZeneca,
MSD, Pharmamar, Tesaro, GSK, Clovis, Advaxis, Pzer, Takeda, Immunogen, Biocad,
Amgen, Novartis and Ellipses, institutional nancial interests for Roche, Pharmamar
and AstraZeneca, and non- nancial interests for ESMO clinical Guidelines (subject
editor for gynecological cancer). XMG, SM, TB, SL, PM, RN, DOD, DQ, MRR, AS, AW,
FP, and CLC: no conicts of interest.
Patient consent for publication Not required.
Provenance and peer review Commissioned; internally peer reviewed.
Data availability statement All data relevant to the study are included in the
article or uploaded as supplementary information.
ORCID iDs
NicoleConcin http:// orcid. org/ 0000- 0002- 9795- 2643
JonathanLedermann http:// orcid. org/ 0000- 0003- 3799- 3539
ChristinaFotopoulou http:// orcid. org/ 0000- 0001- 6375- 9645
DenisQuerleu http:// orcid. org/ 0000- 0002- 3984- 4812
REFERENCES
1 World Health Organization. GLOBOCAN 2018: estimated cancer
incidence, mortality and prevalence worldwide in 2018, 2018.
Available: http:// gco. iarc. fr/ today/ data/ factsheets/ cancers/ 24-
Corpus- uteri- fact- sheet. pdf [Accessed 29 Jul 2020].
2 Sant M, Chirlaque Lopez MD, Agresti R, etal. Survival of
women with cancers of breast and genital organs in Europe
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2020-002230 on 18 December 2020. Downloaded from

30
ConcinN, etal. Int J Gynecol Cancer 2021;31:12–39. doi:10.1136/ijgc-2020-002230
Joint statement
1999–2007: results of the EUROCARE-5 study. Eur J Cancer
2015;51:2191–205.
3 Colombo N, Creutzberg C, Amant F, etal. ESMO- ESGO- ESTRO
consensus conference on endometrial cancer: diagnosis, treatment
and follow- up. Int J Gynecol Cancer 2016;26:2–30.
4 Colombo N, Creutzberg C, Amant F, etal. ESMO- ESGO- ESTRO
consensus conference on endometrial cancer: diagnosis, treatment
and follow- up. Radiother Oncol 2015;117:559–81.
5 Colombo N, Creutzberg C, Amant F, etal. ESMO- ESGO- ESTRO
consensus conference on endometrial cancer: diagnosis, treatment
and follow- up. Ann Oncol 2016;27:16–41.
6 Colombo N, Preti E, Landoni F, etal. Endometrial cancer: ESMO
clinical practice guidelines for diagnosis, treatment and follow- up.
Ann Oncol 2013;24 Suppl 6:vi33–8.
7 Dykewicz CA. Summary of the guidelines for preventing
opportunistic infections among hematopoietic stem cell transplant
recipients. Clin Infect Dis 2001;33:139–44.
8 Ryan NAJ, Glaire MA, Blake D, etal. The proportion of
endometrial cancers associated with Lynch syndrome: a
systematic review of the literature and meta- analysis. Genet Med
2019;21:2167–80.
9 Cho KR, Cooper K, Croce S, etal. International Society of
Gynecological Pathologists (ISGyP) endometrial cancer project:
guidelines from the special techniques and ancillary studies group.
Int J Gynecol Pathol 2019;38 Suppl 1:S114–22.
10 Crosbie EJ, Ryan NAJ, Arends MJ, etal. The Manchester
International Consensus Group recommendations for the
management of gynecological cancers in Lynch syndrome. Genet
Med 2019;21:2390–400.
11 Mills AM, Liou S, Ford JM, etal. Lynch syndrome screening should
be considered for all patients with newly diagnosed endometrial
cancer. Am J Surg Pathol 2014;38:1501–9.
12 Mojtahed A, Schrijver I, Ford JM, etal. A two- antibody mismatch
repair protein immunohistochemistry screening approach for
colorectal carcinomas, skin sebaceous tumors, and gynecologic
tract carcinomas. Mod Pathol 2011;24:1004–14.
13 Shia J, Tang LH, Vakiani E, etal. Immunohistochemistry as rst-
line screening for detecting colorectal cancer patients at risk for
hereditary nonpolyposis colorectal cancer syndrome: a 2- antibody
panel may be as predictive as a 4- antibody panel. Am J Surg
Pathol 2009;33:1639–45.
14 Møller P, Seppälä T, Bernstein I, etal. Cancer incidence and
survival in Lynch syndrome patients receiving colonoscopic and
gynaecological surveillance: rst report from the prospective Lynch
syndrome database. Gut 2017;66:464–72.
15 Ryan NAJ, Morris J, Green K, etal. Association of mismatch
repair mutation with age at cancer onset in Lynch syndrome:
implications for stratied surveillance strategies. JAMA Oncol
2017;3:1702–6.
16 Lachiewicz MP, Kravochuck SE, O'Malley MM, etal. Prevalence
of occult gynecologic malignancy at the time of risk reducing and
nonprophylactic surgery in patients with Lynch syndrome. Gynecol
Oncol 2014;132:434–7.
17 Kandoth C, Schultz N, etal, Cancer Genome Atlas Research
Network. Integrated genomic characterization of endometrial
carcinoma. Nature 2013;497:67–73.
18 Piulats JM, Guerra E, Gil- Martín M, etal. Molecular approaches for
classifying endometrial carcinoma. Gynecol Oncol 2017;145:200–7.
19 Talhouk A, McConechy MK, Leung S, etal. A clinically applicable
molecular- based classication for endometrial cancers. Br J
Cancer 2015;113:299–310.
20 Talhouk A, McConechy MK, Leung S, etal. Conrmation
of ProMisE: a simple, genomics- based clinical classier for
endometrial cancer. Cancer 2017;123:802–13.
21 Stelloo E, Nout RA, Osse EM, etal. Improved risk assessment by
integrating molecular and clinicopathological factors in early- stage
endometrial cancer—combined analysis of the PORTEC cohorts.
Clin Cancer Res 2016;22:4215–24.
22 León- Castillo A, de Boer SM, Powell ME, etal. Molecular
classication of the PORTEC-3 trial for high- risk endometrial
cancer: impact on prognosis and benet from adjuvant therapy. J
Clin Oncol 2020;38:3388–97.
23 León‐Castillo A, Gilvazquez E, Nout R, etal. Clinicopathological
and molecular characterisation of ‘multiple‐classier’ endometrial
carcinomas. J Pathol 2020;250:312–22.
24 Vermij L, Smit V, Nout R, etal. Incorporation of molecular
characteristics into endometrial cancer management.
Histopathology 2020;76:52–63.
25 Church DN, Stelloo E, Nout RA, etal. Prognostic signicance of
POLE proofreading mutations in endometrial cancer. J Natl Cancer
Inst 2015;107.
26 León‐Castillo A, Britton H, McConechy MK, etal. Interpretation
of somatic POLE mutations in endometrial carcinoma. J Pathol
2020;250:323–35.
27 McAlpine J, Leon- Castillo A, Bosse T. The rise of a novel
classication system for endometrial carcinoma; integration of
molecular subclasses. J Pathol 2018;244:538–49.
28 Köbel M, Nelson GS. Letter in response to: McAlpine J, Leon-
Castillo a, Bosse T. the rise of a novel classication system for
endometrial carcinoma; integration of molecular subclasses. J
Pathol 2018; 244: 538-549. J Pathol 2018;245:249–50.
29 Kommoss FKF, Karnezis AN, Kommoss F, etal. L1Cam further
straties endometrial carcinoma patients with no specic molecular
risk prole. Br J Cancer 2018;119:480–6.
30 van der Putten LJM, Visser NCM, van de Vijver K, etal. L1CAM
expression in endometrial carcinomas: an ENITEC collaboration
study. Br J Cancer 2016;115:716–24.
31 Van Gool IC, Stelloo E, Nout RA, etal. Prognostic signicance of
L1CAM expression and its association with mutant p53 expression
in high- risk endometrial cancer. Mod Pathol 2016;29:174–81.
32 Bosse T, Nout RA, Stelloo E, etal. L1 cell adhesion molecule is a
strong predictor for distant recurrence and overall survival in early
stage endometrial cancer: pooled PORTEC trial results. Eur J
Cancer 2014;50:2602–10.
33 WHO Classication of Tumours. Female genital organ tumours,
International agency for research on cancer IARC. 5th edn. Lyon,
2020.
34 Ali A, Black D, Soslow RA. Difculties in assessing the depth of
myometrial invasion in endometrial carcinoma. Int J Gynecol Pathol
2007;26:115–23.
35 Luomaranta A, Leminen A, Loukovaara M. Magnetic resonance
imaging in the assessment of high- risk features of endometrial
carcinoma: a meta- analysis. Int J Gynecol Cancer 2015;25:837–42.
36 Andreano A, Rechichi G, Rebora P, etal. MR diffusion imaging
for preoperative staging of myometrial invasion in patients with
endometrial cancer: a systematic review and meta- analysis. Eur
Radiol 2014;24:1327–38.
37 Das SK, Niu XK, Wang JL, etal. Usefulness of DWI in preoperative
assessment of deep myometrial invasion in patients with
endometrial carcinoma: a systematic review and meta- analysis.
Cancer Imaging 2014;14.
38 Deng L, Wang Q- P, Chen X, etal. The combination of diffusion- and
T2- weighted imaging in predicting deep myometrial invasion of
endometrial cancer. J Comput Assist Tomogr 2015;39:661–73.
39 Alcázar JL, Gastón B, Navarro B, etal. Transvaginal ultrasound
versus magnetic resonance imaging for preoperative assessment
of myometrial inltration in patients with endometrial cancer:
a systematic review and meta- analysis. J Gynecol Oncol
2017;28:e86.
40 Tanaka T, Terai Y, Fujiwara S, etal. Preoperative diffusion- weighted
magnetic resonance imaging and intraoperative frozen sections
for predicting the tumor grade in endometrioid endometrial cancer.
Oncotarget 2018;9:36575–84.
41 Sánchez MF, Causa Andrieu PI, Latapie C, etal. Diagnostic yield
of magnetic resonance imaging and intraoperative frozen section
in the determination of deep myometrial invasion in endometrial
cancer. Radiología 2019;61:315–23.
42 Fasmer KE, Bjørnerud A, Ytre- Hauge S, etal. Preoperative
quantitative dynamic contrast- enhanced MRI and diffusion-
weighted imaging predict aggressive disease in endometrial cancer.
Acta Radiol 2018;59:1010–7.
43 Tauq M, Masroor I, Hussain Z. Diagnostic accuracy of diffusion
weighted magnetic resonance imaging in the detection of
myometrial invasion in endometrial carcinoma. J Coll Physicians
Surg Pak 2016;26:13–17.
44 Christensen JW, Dueholm M, Hansen ES, etal. Assessment of
myometrial invasion in endometrial cancer using three- dimensional
ultrasound and magnetic resonance imaging. Acta Obstet Gynecol
Scand 2016;95:55–64.
45 Arnaiz J, Muñoz A- B, Verna V, etal. Magnetic resonance imaging
for the pre- surgical assessment of endometrial cancer: results in a
routine clinical setting, outside dedicated trials; a cross- sectional
study. Anticancer Res 2016;36:1891–4.
46 Shrivastava S, Barmon D, Kataki AC, etal. Magnetic resonance
imaging in pre- operative staging of endometrial cancer. Indian J
Cancer 2016;53:181–5.
47 Body N, Lavoué V, De Kerdaniel O, etal. Are preoperative histology
and MRI useful for classication of endometrial cancer risk? BMC
Cancer 2016;16:498.
48 Rodríguez- Trujillo A, Martínez- Serrano MJ, Martínez- Román S, etal.
Preoperative assessment of myometrial invasion in endometrial
cancer by 3D ultrasound and diffusion- weighted magnetic
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2020-002230 on 18 December 2020. Downloaded from

31
ConcinN, etal. Int J Gynecol Cancer 2021;31:12–39. doi:10.1136/ijgc-2020-002230
Joint statement
resonance imaging: a comparative study. Int J Gynecol Cancer
2016;26:1105–10.
49 Horváth K, Pete I, Vereczkey I, etal. Evaluation of the accuracy of
preoperative MRI in measuring myometrial inltration in endometrial
carcinoma. Pathol Oncol Res 2014;20:327–33.
50 Nougaret S, Reinhold C, Alsharif SS, etal. Endometrial cancer:
combined MR volumetry and diffusion- weighted imaging for
assessment of myometrial and lymphovascular invasion and tumor
grade. Radiology 2015;276:797–808.
51 Ippolito D, Cadonici A, Bonafni PA, etal. Semiquantitative
perfusion combined with diffusion- weighted MR imaging in pre-
operative evaluation of endometrial carcinoma: results in a group of
57 patients. Magn Reson Imaging 2014;32:464–72.
52 Tanaka T, Terai Y, Ono YJ, etal. Preoperative MRI and
intraoperative frozen section diagnosis of myometrial invasion
in patients with endometrial cancer. Int J Gynecol Cancer
2015;25:879–83.
53 Bonatti M, Stuefer J, Oberhofer N, etal. MRI for local staging
of endometrial carcinoma: is endovenous contrast medium
administration still needed? Eur J Radiol 2015;84:208–14.
54 Karataşlı V, Çakır İ, Şahin H, etal. Can preoperative magnetic
resonance imaging replace intraoperative frozen sectioning in
the evaluation of myometrial invasion for early- stage endometrial
carcinoma? Ginekol Pol 2019;90:128–33.
55 Fujii S, Kido A, Baba T, etal. Subendometrial enhancement and
peritumoral enhancement for assessing endometrial cancer
on dynamic contrast enhanced MR imaging. Eur J Radiol
2015;84:581–9.
56 Yang T, Tian S, Li Y, etal. Magnetic resonance imaging (MRI) and
three- dimensional transvaginal ultrasonography scanning for
preoperative assessment of high risk in women with endometrial
cancer. Med Sci Monit 2019;25:2024–31.
57 Ahmed M, Al- Khafaji JF, Class CA, etal. Can MRI help assess
aggressiveness of endometrial cancer? Clin Radiol 2018;73:833.
e11–833.e18.
58 Sahin H, Sarioglu FC, Bagci M, etal. Preoperative magnetic
resonance volumetry in predicting myometrial invasion,
lymphovascular space invasion, and tumor grade: is it valuable
in International Federation of Gynecology and Obstetrics stage I
endometrial cancer? Int J Gynecol Cancer 2018;28:666–74.
59 Yan B, Zhao T, Liang X, etal. Can the apparent diffusion coefcient
differentiate the grade of endometrioid adenocarcinoma and
the histological subtype of endometrial cancer? Acta Radiol
2018;59:363–70.
60 Zhang L, Liu A, Zhang T, etal. Use of diffusion tensor imaging
in assessing supercial myometrial invasion by endometrial
carcinoma: a preliminary study. Acta Radiol 2015;56:1273–80.
61 Bonatti M, Pedrinolla B, Cybulski AJ, etal. Prediction of histological
grade of endometrial cancer by means of MRI. Eur J Radiol
2018;103:44–50.
62 Tsikouras P, Koukouli Z, Bothou A, etal. Preoperative assessment
in endometrial cancer: is triage for lymphadenectomy possible? J
Buon 2017;22:34–43.
63 Zamani N, Modares Gilani M, Zamani F, etal. Utility of pelvic MRI
and tumor markers HE4 and CA125 to predict depth of myometrial
invasion and cervical involvement in endometrial cancer. J Family
Reprod Health 2015;9:177–83.
64 Bourgioti C, Chatoupis K, Tzavara C, etal. Predictive ability of
maximal tumor diameter on MRI for high- risk endometrial cancer.
Abdom Radiol 2016;41:2484–95.
65 Ytre- Hauge S, Dybvik JA, Lundervold A, etal. Preoperative
tumor texture analysis on MRI predicts high- risk disease and
reduced survival in endometrial cancer. J Magn Reson Imaging
2018;48:1637–47.
66 Thieme SF, Collettini F, Sehouli J, etal. Preoperative evaluation of
myometrial invasion in endometrial carcinoma: prospective intra-
individual comparison of magnetic resonance volumetry, diffusion-
weighted and dynamic contrast- enhanced magnetic resonance
imaging. Anticancer Res 2018;38:4813–7.
67 Deng L, Wang Q- P, Yan R, etal. Combined subjective and
quantitative analysis of magnetic resonance images could improve
the diagnostic performance of deep myometrial invasion in
endometrial cancer. Clin Imaging 2017;43:69–73.
68 Gallego JC, Porta A, Pardo MC, etal. Evaluation of myometrial
invasion in endometrial cancer: comparison of diffusion- weighted
magnetic resonance and intraoperative frozen sections. Abdom
Imaging 2014;39:1021–6.
69 Brocker KA, Radtke JP, Hallscheidt P, etal. Comparison of the
determination of the local tumor extent of primary endometrial cancer
using clinical examination and 3 tesla magnetic resonance imaging
compared to histopathology. Arch Gynecol Obstet 2019;299:1391–8.
70 Goel G, Rajanbabu A, Sandhya CJ, etal. A prospective
observational study evaluating the accuracy of MRI in predicting
the extent of disease in endometrial cancer. Indian J Surg Oncol
2019;10:220–4.
71 Cignini P, Vitale SG, Laganà AS, etal. Preoperative work- up for
denition of lymph node risk involvement in early stage endometrial
cancer: 5- year follow- up. Updates Surg 2017;69:75–82.
72 Soneji ND, Bharwani N, Ferri A, etal. Pre- operative MRI staging of
endometrial cancer in a multicentre cancer network: can we match
single centre study results? Eur Radiol 2018;28:4725–34.
73 Green RW, Valentin L, Alcazar JL, etal. Endometrial cancer off- line
staging using two- dimensional transvaginal ultrasound and three-
dimensional volume contrast imaging: intermethod agreement,
interrater reliability and diagnostic accuracy. Gynecol Oncol
2018;150:438–45.
74 Takeuchi M, Matsuzaki K, Harada M. Evaluating myometrial
invasion in endometrial cancer: comparison of reduced eld- of-
view diffusion- weighted imaging and dynamic contrast- enhanced
MR imaging. MRMS 2018;17:28–34.
75 Ota T, Hori M, Onishi H, etal. Preoperative staging of endometrial
cancer using reduced eld- of- view diffusion- weighted imaging: a
preliminary study. Eur Radiol 2017;27:5225–35.
76 Koplay M, Dogan NU, Erdogan H, etal. Diagnostic efcacy
of diffusion- weighted MRI for pre- operative assessment of
myometrial and cervical invasion and pelvic lymph node
metastasis in endometrial carcinoma. J Med Imaging Radiat Oncol
2014;58:538–46.
77 Woo S, Kim SY, Cho JY, etal. Assessment of deep myometrial
invasion of endometrial cancer on MRI: added value of second-
opinion interpretations by radiologists subspecialized in
gynaecologic oncology. Eur Radiol 2017;27:1877–82.
78 Alves I, Cunha TM. Clinical importance of second- opinion
interpretations by radiologists specializing in gynecologic oncology
at a tertiary cancer center: magnetic resonance imaging for
endometrial cancer staging. Radiol Bras 2018;51:26–31.
79 Masroor I, Rashid S, Afzal S, etal. Diagnostic accuracy of pelvic
MRI for determination of the cervical involvement in endometrial
cancer. J Coll Physicians Surg Pak 2018;27:262–5.
80 Teng F, Zhang Y- F, Wang Y- M, etal. Contrast- enhanced MRI in
preoperative assessment of myometrial and cervical invasion,
and lymph node metastasis: diagnostic value and error
analysis in endometrial carcinoma. Acta Obstet Gynecol Scand
2015;94:266–73.
81 Bhosale P, Ma J, Iyer R, etal. Feasibility of a reduced eld- of- view
diffusion- weighted (rFOV) sequence in assessment of myometrial
invasion in patients with clinical FIGO stage I endometrial cancer. J
Magn Reson Imaging 2016;43:316–24.
82 Lin G, Huang Y- T, Chao A, etal. Endometrial cancer with cervical
stromal invasion: diagnostic accuracy of diffusion- weighted
and dynamic contrast enhanced MR imaging at 3T. Eur Radiol
2017;27:1867–76.
83 Alcazar JL, Pineda L, Martinez- Astorquiza Corral T, etal.
Transvaginal/transrectal ultrasound for assessing myometrial
invasion in endometrial cancer: a comparison of six different
approaches. J Gynecol Oncol 2015;26:201–7.
84 Eriksson LSE, Lindqvist PG, Flöter Rådestad A, etal. Transvaginal
ultrasound assessment of myometrial and cervical stromal invasion
in women with endometrial cancer: interobserver reproducibility
among ultrasound experts and gynecologists. Ultrasound Obstet
Gynecol 2015;45:476–82.
85 Vieillefosse S, Huchon C, Chammings F, etal. Assessment of
different pre and intra- operative strategies to predict the actual
ESMO risk group and to establish the appropriate indication of
lymphadenectomy in endometrial cancer. J Gynecol Obstet Hum
Reprod 2018;47:517–23.
86 Jantarasaengaram S, Praditphol N, Tansathit T, etal. Three-
dimensional ultrasound with volume contrast imaging for
preoperative assessment of myometrial invasion and cervical
involvement in women with endometrial cancer. Ultrasound Obstet
Gynecol 2014;43:569–74.
87 Pineda L, Alcázar JL, Caparrós M, etal. Agreement between
preoperative transvaginal ultrasound and intraoperative
macroscopic examination for assessing myometrial inltration
in low- risk endometrioid carcinoma. Ultrasound Obstet Gynecol
2016;47:369–73.
88 Frühauf F, Zikan M, Semeradova I, etal. The diagnostic accuracy
of ultrasound in assessment of myometrial invasion in endometrial
cancer: subjective assessment versus objective techniques.
Biomed Res Int 2017;2017:1–10.
89 Bollineni VR, Ytre- Hauge S, Bollineni- Balabay O, etal. High
diagnostic value of 18F- FDG PET/CT in endometrial cancer:
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2020-002230 on 18 December 2020. Downloaded from

32
ConcinN, etal. Int J Gynecol Cancer 2021;31:12–39. doi:10.1136/ijgc-2020-002230
Joint statement
systematic review and meta- analysis of the literature. J Nucl Med
2016;57:879–85.
90 Legros M, Margueritte F, Tardieu A, etal. Para- aortic lymph node
invasion in high- risk endometrial cancer: performance of (18)FDG
PET- CT. Anticancer Res 2019;39:619–25.
91 Kim HJ, Cho A, Yun M, etal. Comparison of FDG PET/CT and
MRI in lymph node staging of endometrial cancer. Ann Nucl Med
2016;30:104–13.
92 Chung HH, Cheon GJ, Kim HS, etal. Preoperative PET/CT
standardized FDG uptake values of pelvic lymph nodes as a
signicant prognostic factor in patients with endometrial cancer.
Eur J Nucl Med Mol Imaging 2014;41:1793–9.
93 Atakul BK, Taşkın S, Soydal Ç, etal. Preoperative 18F-
uorodeoxyglucose positron emission tomography/CT in prediction
of uterine risk factors and lymph node metastasis: an analysis of
111 endometrioid endometrial cancer patients. Gynecol Obstet
Invest 2017;82:340–8.
94 Kulkarni R, Bhat RA, Dhakharia V, etal. Role of positron emission
tomography/computed tomography in preoperative assessment of
carcinoma endometrium—a retrospective analysis. Indian J Surg
Oncol 2019;10:225–31.
95 Tanaka T, Terai Y, Yamamoto K, etal. The diagnostic accuracy of
uorodeoxyglucose- positron emission tomography/computed
tomography and sentinel node biopsy in the prediction of pelvic
lymph node metastasis in patients with endometrial cancer.
Medicine 2018;97:e12522.
96 Bese T, Sal V, Demirkiran F, etal. The combination of preoperative
uorodeoxyglucose positron emission tomography/computed
tomography and sentinel lymph node mapping in the surgical
management of endometrioid endometrial cancer. Int J Gynecol
Cancer 2016;26:1228–38.
97 Park J- Y, Lee JJ, Choi HJ, etal. The value of preoperative positron
emission tomography/computed tomography in node- negative
endometrial cancer on magnetic resonance imaging. Ann Surg
Oncol 2017;24:2303–10.
98 Gülseren V, Kocaer M, Çelikkol Güngördük Ö, etal. Is the
measurement of the size of uterine lesions with positron emission
tomography consistent in pre- and postmenopausal periods in
endometrioid- type endometrial cancer? Turk J Obstet Gynecol
2018;15:60–4.
99 Gholkar NS, Saha SC, Prasad G, etal. The accuracy of integrated
[(18)F] uorodeoxyglucose- positron emission tomography/
computed tomography in detection of pelvic and para- aortic nodal
metastasis in patients with high risk endometrial cancer. World J
Nucl Med 2014;13:170–7.
100 Mayoral M, Paredes P, Domènech B, etal.
18
F- FDG PET/CT and
sentinel lymph node biopsy in the staging of patients with cervical
and endometrial cancer. Role of dual- time- point imaging. Rev Esp
Med Nucl Imagen Mol 2017;36:20–6.
101 Ghooshkhanei H, Treglia G, Sabouri G, etal. Risk stratication
and prognosis determination using 18F- FDG PET imaging in
endometrial cancer patients: a systematic review and meta-
analysis. Gynecol Oncol 2014;132:669–76.
102 Dai S, Nahas S, Murphy JK, etal. Impact and cost of preoperative
computed tomography imaging on the management of patients
diagnosed with high‐grade endometrial cancer. Int J Gynecol
Obstet 2019;145:219–24.
103 Bogani G, Gostout BS, Dowdy SC, etal. Clinical utility of
preoperative computed tomography in patients with endometrial
cancer. Int J Gynecol Cancer 2017;27:1685–93.
104 Janda M, Gebski V, Davies LC, etal. Effect of total laparoscopic
hysterectomy vs total abdominal hysterectomy on disease- free
survival among women with stage I endometrial cancer. JAMA
2017;317:1224–33.
105 Walker JL, Piedmonte MR, Spirtos NM, etal. Recurrence and
survival after random assignment to laparoscopy versus
laparotomy for comprehensive surgical staging of uterine
cancer: Gynecologic Oncology Group LAP2 study. JCO
2012;30:695–700.
106 Togami S, Kawamura T, Fukuda M, etal. Learning curve and
surgical outcomes for laparoscopic surgery, including pelvic
lymphadenectomy, for early stage endometrial cancer. Jpn J Clin
Oncol 2019;49:521–4.
107 Deura I, Shimada M, Azuma Y, etal. Comparison of laparoscopic
surgery and conventional laparotomy for surgical staging of
patients with presumed low- risk endometrial cancer: the current
state of Japan. Taiwan J Obstet Gynecol 2019;58:99–104.
108 Ghazali WHW, Jamil S, Sharin I. Laparoscopic versus laparotomy:
Staging surgery for endometrial cancer – Malaysia’s early
experience. Gynecol Minim Invasive Ther 2019;8:25–9.
109 Vardar MA, Gulec UK, Guzel AB, etal. Laparoscopic surgery for
low, intermediate and high- risk endometrial cancer. J Gynecol
Oncol 2019;30:e24.
110 Pookunju AP, Ayyappan S. Technique of laparoscopic hysterectomy
and pelvic lymphadenectomy for endometrial cancer. Indian J Surg
Oncol 2018;9:290–3.
111 Wollinga T, Ezendam NPM, Eggink FA, etal. Implementation of
laparoscopic hysterectomy for endometrial cancer over the past
decade. Gynecol Surg 2018;15:7.
112 Van den Bosch A, Mertens H. Implementation of laparoscopic
surgery for endometrial cancer: work in progress. Facts Views Vis
Obgyn 2016;8:23–30.
113 Chu L- H, Chang W- C, Sheu B- C. Comparison of the laparoscopic
versus conventional open method for surgical staging of
endometrial carcinoma. Taiwan J Obstet Gynecol 2016;55:188–92.
114 Favero G, Anton C, Le X, etal. Oncologic safety of laparoscopy in
the surgical treatment of type II endometrial cancer. Int J Gynecol
Cancer 2016;26:1673–8.
115 Bennich G, Rudnicki M, Lassen PD. Laparoscopic surgery for early
endometrial cancer. Acta Obstet Gynecol Scand 2016;95:894–900.
116 Lee C- L, Kusunoki S, Huang K- G, etal. Long- term survival
outcomes of laparoscopic staging surgery in treating endometrial
cancer: 20 years of follow- up. Taiwan J Obstet Gynecol
2016;55:545–51.
117 Berretta R, Gizzo S, Noventa M, etal. Quality of life in patients
affected by endometrial cancer: comparison among laparotomy,
laparoscopy and vaginal approach. Pathol Oncol Res
2015;21:811–6.
118 Yin X, Shi M, Xu J, etal. Perioperative and long- term outcomes of
laparoscopy and laparotomy for endometrial carcinoma. Int J Clin
Exp Med 2015;8:19093–9.
119 Kroft J, Li Q, Saskin R, etal. Trends over time in the use of
laparoscopic hysterectomy for the treatment of endometrial cancer.
Gynecol Oncol 2015;138:536–41.
120 Pawłowicz PS, Ajdacka U. The role of laparoscopy in the surgical
treatment of endometrial cancer. Wiitm 2015;1:44–8.
121 Gao H, Zhang Z. Laparoscopy versus laparotomy in the treatment
of high- risk endometrial cancer: a propensity score matching
analysis. Medicine 2015;94:e1245.
122 Şenol T, Polat M, Şanverdi I, etal. Laparoscopic staging of
endometrial cancer: does it have any impact on survival? Turk J
Obstet Gynecol 2015;12:139–43.
123 Palomba S, Ghezzi F, Falbo A, etal. Conversion in endometrial
cancer patients scheduled for laparoscopic staging: a large
multicenter analysis: conversions and endometrial cancer. Surg
Endosc 2014;28:3200–9.
124 Lee C- L, Huang K- G, Wu P- J, etal. Long- term survival outcome of
laparoscopic staging surgery for endometrial cancer in Taiwanese
experience. Taiwan J Obstet Gynecol 2014;53:57–61.
125 Terai Y, Tanaka T, Sasaki H, etal. Total laparoscopic modied
radical hysterectomy with lymphadenectomy for endometrial
cancer compared with laparotomy. J Obstet Gynaecol Res
2014;40:570–5.
126 Koskas M, Jozwiak M, Fournier M, etal. Long- term oncological
safety of minimally invasive surgery in high- risk endometrial cancer.
Eur J Cancer 2016;65:185–91.
127 Uccella S, Bonzini M, Palomba S, etal. Laparoscopic vs. open
treatment of endometrial cancer in the elderly and very elderly: an
age- stratied multicenter study on 1606 women. Gynecol Oncol
2016;141:211–7.
128 Bogani G, Cromi A, Uccella S, etal. Perioperative and long- term
outcomes of laparoscopic, open abdominal, and vaginal surgery for
endometrial cancer in patients aged 80 years or older. Int J Gynecol
Cancer 2014;24:894–900.
129 Baek M- H, Lee S- W, Park J- Y, etal. Feasibility and safety of
laparoscopic surgery for obese Korean women with endometrial
cancer: long- term results at a single institution. J Korean Med Sci
2014;29:1536–43.
130 Bogani G, Cromi A, Uccella S, etal. Laparoscopic staging
in women older than 75 years with early- stage endometrial
cancer: comparison with open surgical operation. Menopause
2014;21:945–51.
131 Freeman AH, Barrie A, Lyon L, etal. Venous thromboembolism
following minimally invasive surgery among women with
endometrial cancer. Gynecol Oncol 2016;142:267–72.
132 Raventós- Tato RM, de la Torre- Fernández de Vega J, Sánchez-
Iglesias JL, etal. Surgical approaches in women with endometrial
cancer with a body mass index greater than 35 kg/m
2
. J Obstet
Gynaecol Res 2019;45:195–202.
133 Bishop EA, Java JJ, Moore KN, etal. Surgical outcomes among
elderly women with endometrial cancer treated by laparoscopic
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2020-002230 on 18 December 2020. Downloaded from

33
ConcinN, etal. Int J Gynecol Cancer 2021;31:12–39. doi:10.1136/ijgc-2020-002230
Joint statement
hysterectomy: a NRG/Gynecologic Oncology Group study. Am J
Obstet Gynecol 2018;218:109.e1–109.e11.
134 Casarin J, Multinu F, Ubl DS, etal. Adoption of minimally invasive
surgery and decrease in surgical morbidity for endometrial cancer
treatment in the United States. Obstet Gynecol 2018;131:304–11.
135 Ee WW, Nellore V, McMullen W, etal. Laparoscopic hysterectomy
for endometrial cancer: impact of age on clinical outcomes. J
Obstet Gynaecol 2018;38:734.
136 Singh S, Swarer K, Resnick K. Longer operative time is associated
with increased post- operative complications in patients undergoing
minimally- invasive surgery for endometrial cancer. Gynecol Oncol
2017;147:554–7.
137 Bregar AJ, Melamed A, Diver E, etal. Minimally invasive staging
surgery in women with early- stage endometrial cancer: analysis of
the National Cancer Data Base. Ann Surg Oncol 2017;24:1677–87.
138 Monterossi G, Ghezzi F, Vizza E, etal. Minimally invasive approach
in type II endometrial cancer: is it wise and safe? J Minim Invasive
Gynecol 2017;24:438–45.
139 Barber EL, Gehrig PA, Clarke- Pearson DL. Venous
thromboembolism in minimally invasive compared with
open hysterectomy for endometrial cancer. Obstet Gynecol
2016;128:121–6.
140 Pulman KJ, Dason ES, Philp L, etal. Comparison of three surgical
approaches for staging lymphadenectomy in high- risk endometrial
cancer. Int J Gynecol Obstet 2017;136:315–9.
141 Marcos- Sanmartín J, López Fernández JA, Sánchez- Payá J,
etal. Does the type of surgical approach and the use of uterine
manipulators inuence the disease- free survival and recurrence
rates in early- stage endometrial cancer? Int J Gynecol Cancer
2016;26:1722–6.
142 Tanaka T, Terai Y, Hayashi S, etal. Comparison between
laparoscopy and laparotomy in systematic para- aortic
lymphadenectomy for patients with endometrial cancer: a
retrospective multicenter study. J Gynecol Surg 2017;33:105–10.
143 Galaal K, Donkers H, Bryant A, etal. Laparoscopy versus
laparotomy for the management of early stage endometrial cancer.
Cochrane Database Syst Rev 2018;10.
144 Asher R, Obermair A, Janda M, etal. Disease- free and survival
outcomes for total laparoscopic hysterectomy compared with total
abdominal hysterectomy in early- stage endometrial carcinoma: a
meta- analysis. Int J Gynecol Cancer 2018;28:529–38.
145 Mahajan V. Prospective nonrandomized comparative study of
laparoscopic versus open surgical staging for endometrial cancer in
India. Indian J Surg Oncol 2018;9:133–40.
146 Jørgensen SL, Mogensen O, Wu C, etal. Nationwide introduction
of minimally invasive robotic surgery for early- stage endometrial
cancer and its association with severe complications. JAMA Surg
2019;154:530.
147 Kyrgiou M, Swart AM, Qian W, etal. A comparison of outcomes
following laparoscopic and open hysterectomy with or without
lymphadenectomy for presumed early- stage endometrial cancer:
results from the Medical Research Council ASTEC trial. Int J
Gynecol Cancer 2015;25:1424–36.
148 Park DA, Lee DH, Kim SW, etal. Comparative safety and
effectiveness of robot- assisted laparoscopic hysterectomy
versus conventional laparoscopy and laparotomy for endometrial
cancer: a systematic review and meta- analysis. Eur J Surg Oncol
2016;42:1303–14.
149 Ran L, Jin J, Xu Y, etal. Comparison of robotic surgery with
laparoscopy and laparotomy for treatment of endometrial cancer: a
meta- analysis. PLoS One 2014;9:e108361.
150 Nevis IF, Vali B, Higgins C, etal. Robot- assisted hysterectomy for
endometrial and cervical cancers: a systematic review. J Robot
Surg 2017;11:1–16.
151 Lundin ES, Wodlin NB, Nilsson L, etal. A prospective randomized
assessment of quality of life between open and robotic
hysterectomy in early endometrial cancer. Int J Gynecol Cancer
2019. doi:10.1136/ijgc-2019-000285. [Epub ahead of print: 28 Mar
2019].
152 Herling SF, Møller AM, Palle C, etal. Robotic- assisted laparoscopic
hysterectomy for women with endometrial cancer. Dan Med J
2017;64.
153 Uccella S, Bonzini M, Palomba S, etal. Impact of obesity on
surgical treatment for endometrial cancer: a multicenter study
comparing laparoscopy vs open surgery, with propensity- matched
analysis. J Minim Invasive Gynecol 2016;23:53–61.
154 Corrado G, Mereu L, Bogliolo S, etal. Robotic single site staging
in endometrial cancer: a multi- institution study. Eur J Surg Oncol
2016;42:1506–11.
155 Backes FJ, El Naggar AC, Farrell MR, etal. Perioperative outcomes
for laparotomy compared to robotic surgical staging of endometrial
cancer in the elderly: a retrospective cohort. Int J Gynecol Cancer
2016;26:1717–21.
156 Guy MS, Sheeder J, Behbakht K, etal. Comparative outcomes
in older and younger women undergoing laparotomy or robotic
surgical staging for endometrial cancer. Am J Obstet Gynecol
2016;214:350.e1–350.e10.
157 Herling SF, Havemann MC, Palle C, etal. Robotic- Assisted
laparoscopic hysterectomy seems safe in women with early- stage
endometrial cancer. Dan Med J 2015;62:A5109.
158 Beck TL, Schiff MA, Goff BA, etal. Robotic, laparoscopic, or open
hysterectomy: surgical outcomes by approach in endometrial
cancer. J Minim Invasive Gynecol 2018;25:986–93.
159 Doo DW, Guntupalli SR, Corr BR, etal. Comparative surgical
outcomes for endometrial cancer patients 65 years old or
older staged with robotics or laparotomy. Ann Surg Oncol
2015;22:3687–94.
160 Park HK, Helenowski IB, Berry E, etal. A comparison of survival
and recurrence outcomes in patients with endometrial cancer
undergoing robotic versus open surgery. J Minim Invasive Gynecol
2015;22:961–7.
161 Feuer GA, Lakhi N, Woo A, etal. Robotic surgery for staging of
serous papillary and clear cell carcinoma of the endometrium. Int J
Med Robotics Comput Assist Surg 2014;10:306–13.
162 Pant A, Schink J, Lurain J. Robotic surgery compared with
laparotomy for high- grade endometrial cancer. J Robot Surg
2014;8:163–7.
163 Safdieh J, Lee Y- C, Wong A, etal. A comparison of outcomes
between open hysterectomy and robotic- assisted hysterectomy
for endometrial cancer using the National Cancer Database. Int J
Gynecol Cancer 2017;27:1508–16.
164 Wright JD, Burke WM, Tergas AI, etal. Comparative effectiveness
of minimally invasive hysterectomy for endometrial cancer. JCO
2016;34:1087–96.
165 Barraez D, Godoy H, McElrath T, etal. Low incidence of port- site
metastasis after robotic assisted surgery for endometrial cancer
staging: descriptive analysis. J Robot Surg 2015;9:91–5.
166 Yoon A, Yoo H- N, Lee Y- Y, etal. Robotic single- port hysterectomy,
adnexectomy, and lymphadenectomy in endometrial cancer. J
Minim Invasive Gynecol 2015;22:322.
167 Geppert B, Persson J. Robotic infrarenal paraaortic and pelvic
nodal staging for endometrial cancer: feasibility and lymphatic
complications. Acta Obstet Gynecol Scand 2015;94:1074–81.
168 Damiani GR, Turoli D, Cormio G, etal. Robotic approach using
simple and radical hysterectomy for endometrial cancer with long-
term follow- up evaluation. Int J Med Robotics Comput Assist Surg
2016;12:109–13.
169 Bige O, Demir A, Saatli B, etal. Laparoscopy versus laparotomy
for the management of endometrial carcinoma in morbidly obese
patients: a prospective study. J Turkish German Gynecol Assoc
2015;16:164–9.
170 Salehi S, Åvall- Lundqvist E, Legerstam B, etal. Robot- assisted
laparoscopy versus laparotomy for infrarenal paraaortic
lymphadenectomy in women with high- risk endometrial cancer: a
randomised controlled trial. Eur J Cancer 2017;79:81–9.
171 Salehi S, Brandberg Y, Åvall- Lundqvist E, etal. Long- term
quality of life after comprehensive surgical staging of high- risk
endometrial cancer – results from the RASHEC trial. Acta Oncol
2018;57:1671–6.
172 Signorelli M, Lissoni AA, Cormio G, etal. Modied radical
hysterectomy versus extrafascial hysterectomy in the treatment of
stage I endometrial cancer: results from the ILIADE randomized
study. Ann Surg Oncol 2009;16:3431–41.
173 Kaban A, Topuz S, Erdem B, etal. Is omentectomy necessary
for non- endometrioid endometrial cancer. Gynecol Obstet Invest
2018;83:482–6.
174 Joo WD, Schwartz PE, Rutherford TJ, etal. Microscopic omental
metastasis in clinical stage I endometrial cancer: a meta- analysis.
Ann Surg Oncol 2015;22:3695–700.
175 Ross MS, Elishaev E, Berger JL, etal. Prognostic signicance of
omental disease and the role of omental sampling in patients with
uterine carcinosarcoma. Int J Gynecol Cancer 2018;28:254–9.
176 Lee B, Suh DH, Kim K, etal. Inuence of positive peritoneal
cytology on prognostic factors and survival in early- stage
endometrial cancer: a systematic review and meta- analysis. Jpn J
Clin Oncol 2016;46:711–7.
177 Matsuo K, Yabuno A, Hom MS, etal. Signicance of
abnormal peritoneal cytology on survival of women with
stage I–II endometrioid endometrial cancer. Gynecol Oncol
2018;149:301–9.
178 Seagle B- LL, Alexander AL, Lantsman T, etal. Prognosis and
treatment of positive peritoneal cytology in early endometrial
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2020-002230 on 18 December 2020. Downloaded from

34
ConcinN, etal. Int J Gynecol Cancer 2021;31:12–39. doi:10.1136/ijgc-2020-002230
Joint statement
cancer: matched cohort analyses from the National Cancer
Database. Am J Obstet Gynecol 2018;218:329.e1–329.e15.
179 Bogani G, Murgia F, Ditto A, etal. Sentinel node mapping vs.
lymphadenectomy in endometrial cancer: a systematic review and
meta- analysis. Gynecol Oncol 2019;153:676–83.
180 Leitao MM. Sentinel lymph node mapping in patients with
endometrial carcinoma: less can be more. Curr Obstet Gynecol
Rep 2016;5:279–85.
181 Rossi EC, Kowalski LD, Scalici J, etal. A comparison of sentinel
lymph node biopsy to lymphadenectomy for endometrial cancer
staging (FIRES trial): a multicentre, prospective, cohort study.
Lancet Oncol 2017;18:384–92.
182 Persson J, Salehi S, Bollino M, etal. Pelvic Sentinel lymph node
detection in High- Risk Endometrial Cancer (SHREC- trial)—the nal
step towards a paradigm shift in surgical staging. Eur J Cancer
2019;116:77–85.
183 Daraï E, Dubernard G, Bats A- S, etal. Sentinel node biopsy for the
management of early stage endometrial cancer: long- term results
of the SENTI- ENDO study. Gynecol Oncol 2015;136:54–9.
184 Renz M, Marjon N, Devereaux K, etal. Immediate intraoperative
sentinel lymph node analysis by frozen section is predictive of
lymph node metastasis in endometrial cancer. J Robot Surg
2020;14:35–40.
185 How JA, O'Farrell P, Amajoud Z, etal. Sentinel lymph node
mapping in endometrial cancer: a systematic review and meta-
analysis. Minerva Ginecol 2018;70:194–214.
186 Lin H, Ding Z, Kota VG, etal. Sentinel lymph node mapping in
endometrial cancer: a systematic review and meta- analysis.
Oncotarget 2017;8:46601–10.
187 Staley A, Sullivan SA, Rossi EC. Sentinel lymph node technique in
endometrial cancer. Obstet Gynecol Surv 2017;72:289–95.
188 Tschernichovsky R, Diver EJ, Schorge JO, etal. The role of
lymphadenectomy versus sentinel lymph node biopsy in early-
stage endometrial cancer. Am J Clin Oncol 2016;39:516–21.
189 Bodurtha Smith AJ, Fader AN, Tanner EJ. Sentinel lymph node
assessment in endometrial cancer: a systematic review and meta-
analysis. Am J Obstet Gynecol 2017;216:459–76.
190 Wang L, Liu F. Meta- analysis of laparoscopy sentinel lymph
node mapping in endometrial cancer. Arch Gynecol Obstet
2018;298:505–10.
191 Baiocchi G, Mantoan H, Kumagai LY, etal. The impact of sentinel
node- mapping in staging high- risk endometrial cancer. Ann Surg
Oncol 2017;24:3981–7.
192 Tanner E, Puechl A, Levinson K, etal. Use of a novel sentinel
lymph node mapping algorithm reduces the need for pelvic
lymphadenectomy in low- grade endometrial cancer. Gynecol Oncol
2017;147:535–40.
193 Martinelli F, Ditto A, Signorelli M, etal. Sentinel node mapping in
endometrial cancer following hysteroscopic injection of tracers:
a single center evaluation over 200 cases. Gynecol Oncol
2017;146:525–30.
194 Buda A, Di Martino G, Restaino S, etal. The impact on survival of
two different staging strategies in apparent early stage endometrial
cancer comparing sentinel lymph nodes mapping algorithm and
selective lymphadenectomy: an Italian retrospective analysis of two
reference centers. Gynecol Oncol 2017;147:528–34.
195 Yamagami W, Susumu N, Kataoka F, etal. A comparison of dye
versus uorescence methods for sentinel lymph node mapping in
endometrial cancer. Int J Gynecol Cancer 2017;27:1517–24.
196 Touhami O, Grégoire J, Renaud M- C, etal. Performance of sentinel
lymph node (SLN) mapping in high- risk endometrial cancer.
Gynecol Oncol 2017;147:549–53.
197 Papadia A, Buda A, Gasparri ML, etal. The impact of different
doses of indocyanine green on the sentinel lymph- node mapping
in early stage endometrial cancer. J Cancer Res Clin Oncol
2018;144:2187–91.
198 Eoh KJ, Lee YJ, Kim H- S, etal. Two- step sentinel lymph node
mapping strategy in endometrial cancer staging using uorescent
imaging: a novel sentinel lymph node tracer injection procedure.
Surg Oncol 2018;27:514–9.
199 Ducie JA, Eriksson AGZ, Ali N, etal. Comparison of a
sentinel lymph node mapping algorithm and comprehensive
lymphadenectomy in the detection of stage IIIC endometrial
carcinoma at higher risk for nodal disease. Gynecol Oncol
2017;147:541–8.
200 Tanner EJ, Ojalvo L, Stone RL, etal. The utility of sentinel lymph
node mapping in high- grade endometrial cancer. Int J Gynecol
Cancer 2017;27:1416–21.
201 How J, Gauthier C, Abitbol J, etal. Impact of sentinel lymph node
mapping on recurrence patterns in endometrial cancer. Gynecol
Oncol 2017;144:503–9.
202 Papadia A, Zapardiel I, Bussi B, etal. Sentinel lymph node mapping
in patients with stage I endometrial carcinoma: a focus on bilateral
mapping identication by comparing radiotracer Tc99m with blue
dye versus indocyanine green uorescent dye. J Cancer Res Clin
Oncol 2017;143:475–80.
203 Tanaka T, Terai Y, Fujiwara S, etal. The detection of sentinel
lymph nodes in laparoscopic surgery can eliminate systemic
lymphadenectomy for patients with early stage endometrial cancer.
Int J Clin Oncol 2018;23:305–13.
204 Buda A, Gasparri ML, Puppo A, etal. Lymph node evaluation
in high- risk early stage endometrial cancer: a multi- institutional
retrospective analysis comparing the sentinel lymph node (SLN)
algorithm and SLN with selective lymphadenectomy. Gynecol
Oncol 2018;150:261–6.
205 Buda A, Bussi B, Di Martino G, etal. Sentinel lymph node mapping
with near- infrared uorescent imaging using indocyanine green: a
new tool for laparoscopic platform in patients with endometrial and
cervical cancer. J Minim Invasive Gynecol 2016;23:265–9.
206 Buda A, Di Martino G, Vecchione F, etal. Optimizing strategies
for sentinel lymph node mapping in early- stage cervical and
endometrial cancer: comparison of real- time uorescence with
indocyanine green and methylene blue. Int J Gynecol Cancer
2015;25:1513–8.
207 Signorelli M, Crivellaro C, Buda A, etal. Staging of high- risk
endometrial cancer with PET/CT and sentinel lymph node mapping.
Clin Nucl Med 2015;40:780–5.
208 Rajanbabu A, Venkatesan R, Chandramouli S, etal. Sentinel
node detection in endometrial cancer using indocyanine green
and uorescence imaging- a case report. Ecancermedicalscience
2015;9:549.
209 Surynt E, Reinholz- Jaskolska M, Bidzinski M. Laparoscopic sentinel
lymph node mapping after cervical injection of indocyanine green
for endometrial cancer – preliminary report. Wiitm 2015;3:406–12.
210 Chen C- H, Chen H- H, Liu W- M. Detection of sentinel lymph
node mapping using indocyanine green in the management
of endometrial cancer: a pilot study. J Minim Invasive Gynecol
2015;22:S239.
211 Plante M, Touhami O, Trinh X- B, etal. Sentinel node mapping with
indocyanine green and endoscopic near- infrared uorescence
imaging in endometrial cancer. A pilot study and review of the
literature. Gynecol Oncol 2015;137:443–7.
212 Sinno AK, Fader AN, Roche KL, etal. A comparison of colorimetric
versus uorometric sentinel lymph node mapping during robotic
surgery for endometrial cancer. Gynecol Oncol 2014;134:281–6.
213 Blakely M, Liu Y, Rahaman J, etal. Sentinel lymph node ultra-
staging as a supplement for endometrial cancer intraoperative
frozen section deciencies. Int J Gynecol Pathol 2019;38:52–8.
214 Multinu F, Casarin J, Cappuccio S, etal. Ultrastaging of negative
pelvic lymph nodes to decrease the true prevalence of isolated
paraaortic dissemination in endometrial cancer. Gynecol Oncol
2019;154:60–4.
215 Gorostidi M, Villalain C, Ruiz R, etal. Maximizing sentinel lymph
node detection: aortic sentinel lymph node detection in endometrial
cancer. J Minim Invasive Gynecol 2019;26:23–4.
216 Taşkin S, Altin D, Şükür YE, etal. Extrapelvic sentinel lymph nodes
in endometrial cancer patients with unmapped pelvic side: a brief
report. Int J Gynecol Cancer 2018;28:700–3.
217 Fernandez- Prada S, Delgado- Sanchez E, De Santiago J, etal.
Laparoscopic sentinel node biopsy using real- time 3- dimensional
single- photon emission computed tomographic guidance in
endometrial cancer. J Minim Invasive Gynecol 2015;22:1075–8.
218 Ruiz R, Gorostidi M, Jaunarena I, etal. Sentinel node biopsy in
endometrial cancer with dual cervical and fundal indocyanine green
injection. Int J Gynecol Cancer 2018;28:139–44.
219 Euscher E, Sui D, Soliman P, etal. Ultrastaging of sentinel lymph
nodes in endometrial carcinoma according to use of 2 different
methods. Int J Gynecol Pathol 2018;37:242–51.
220 Schlappe BA, Weaver AL, Ducie JA, etal. Multicenter study
comparing oncologic outcomes between two nodal assessment
methods in patients with deeply invasive endometrioid
endometrial carcinoma: a sentinel lymph node algorithm versus a
comprehensive pelvic and paraaortic lymphadenectomy. Gynecol
Oncol 2018;151:235–42.
221 Buda A, Restaino S, Di Martino G, etal. The impact of the type of
nodal assessment on prognosis in patients with high- intermediate
and high- risk ESMO/ESGO/ESTRO group endometrial cancer. A
multicenter Italian study. Eur J Surg Oncol 2018;44:1562–7.
222 Mendivil AA, Abaid LN, Brown JV, etal. The safety and feasibility of
minimally invasive sentinel lymph node staging using indocyanine
green in the management of endometrial cancer. Eur J Obstet
Gynecol Reprod Biol 2018;224:29–32.
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2020-002230 on 18 December 2020. Downloaded from

35
ConcinN, etal. Int J Gynecol Cancer 2021;31:12–39. doi:10.1136/ijgc-2020-002230
Joint statement
223 Restaino S, Ronsini C, Finelli A, etal. Role of blue dye for sentinel
lymph node detection in early endometrial cancer. Gynecol Surg
2017;14:23.
224 Sinno AK, Peijnenburg E, Fader AN, etal. Reducing overtreatment:
a comparison of lymph node assessment strategies for endometrial
cancer. Gynecol Oncol 2016;143:281–6.
225 Naoura I, Canlorbe G, Bendifallah S, etal. Relevance of sentinel
lymph node procedure for patients with high- risk endometrial
cancer. Gynecol Oncol 2015;136:60–4.
226 Papadia A, Gasparri ML, Radan AP, etal. Retrospective validation
of the laparoscopic ICG SLN mapping in patients with grade 3
endometrial cancer. J Cancer Res Clin Oncol 2018;144:1385–93.
227 Papadia A, Gasparri ML, Siegenthaler F, etal. FIGO stage IIIC
endometrial cancer identication among patients with complex
atypical hyperplasia, grade 1 and 2 endometrioid endometrial
cancer: laparoscopic indocyanine green sentinel lymph node
mapping versus frozen section of the uterus, why get around the
problem? J Cancer Res Clin Oncol 2017;143:491–7.
228 Ghezzi F, Casarin J, Uccella S. Mini- laparoscopic sentinel node
detection in endometrial cancer: further reducing invasiveness for
patients with early- stage disease. Ann Surg Oncol 2015;22:S342.
229 Montero Macias R, Balaya V, Bonsang- Kitzis H, etal. Precaval
positive sentinel lymph node with bilateral negative pelvic sentinel
lymph node in low- risk endometrial cancer patient. J Gynecol
Obstet Hum Reprod 2019;48.
230 Brugger S, Hamann M, Mosner M, etal. Endometrial cancer- how
many patients could benet from sentinel lymph node dissection?
World J Surg Oncol 2018;16:95.
231 Kataoka F, Susumu N, Yamagami W, etal. The importance of para-
aortic lymph nodes in sentinel lymph node mapping for endometrial
cancer by using hysteroscopic radio- isotope tracer injection
combined with subserosal dye injection: prospective study.
Gynecol Oncol 2016;140:400–4.
232 Backes FJ, Cohen D, Salani R, etal. Prospective clinical trial of
robotic sentinel lymph node assessment with isosulfane blue (ISB)
and indocyanine green (ICG) in endometrial cancer and the impact
of ultrastaging (NCT01818739). Gynecol Oncol 2019;153:496–9.
233 Togami S, Kawamura T, Fukuda M, etal. Prospective study of
sentinel lymph node mapping for endometrial cancer. Int J Gynecol
Obstet 2018;143:313–8.
234 Rajanbabu A, Agarwal R. A prospective evaluation of the sentinel
node mapping algorithm in endometrial cancer and correlation of
its performance against endometrial cancer risk subtypes. Eur J
Obstet Gynecol Reprod Biol 2018;224:77–80.
235 Farzaneh F, Moridi A, Azizmohammadi Z, etal. Value of sentinel
lymph node (SLN) mapping and biopsy using combined
intracervical radiotracers and blue dye injections for endometrial
cancer. Asian Pac J Cancer Prev 2017;18:431–5.
236 Holloway RW, Ahmad S, Kendrick JE, etal. A prospective cohort
study comparing colorimetric and uorescent imaging for sentinel
lymph node mapping in endometrial cancer. Ann Surg Oncol
2017;24:1972–9.
237 Soliman PT, Westin SN, Dioun S, etal. A prospective validation
study of sentinel lymph node mapping for high- risk endometrial
cancer. Gynecol Oncol 2017;146:234–9.
238 Frati A, Ballester M, Dubernard G, etal. Contribution of
lymphoscintigraphy for sentinel lymph node biopsy in women with
early stage endometrial cancer: results of the SENTI- ENDO study.
Ann Surg Oncol 2015;22:1980–6.
239 Hagen B, Valla M, Aune G, etal. Indocyanine green uorescence
imaging of lymph nodes during robotic- assisted laparoscopic
operation for endometrial cancer. A prospective validation study
using a sentinel lymph node surgical algorithm. Gynecol Oncol
2016;143:479–83.
240 Geppert B, Lönnerfors C, Bollino M, etal. Sentinel lymph node
biopsy in endometrial cancer—feasibility, safety and lymphatic
complications. Gynecol Oncol 2018;148:491–8.
241 Zuo J, Wu LY, Cheng M, etal. Comparison study of laparoscopic
sentinel lymph node mapping in endometrial carcinoma using
carbon nanoparticles and lymphatic pathway verication. J Minim
Invasive Gynecol 2019;26:1125–32.
242 Accorsi GS, Paiva LL, Schmidt R, etal. Sentinel lymph node
mapping vs systematic lymphadenectomy for endometrial cancer:
surgical morbidity and lymphatic complications. J Minim Invasive
Gynecol 2020;27:938–45.
243 Kim CH, Khoury- Collado F, Barber EL, etal. Sentinel lymph node
mapping with pathologic ultrastaging: a valuable tool for assessing
nodal metastasis in low- grade endometrial cancer with supercial
myoinvasion. Gynecol Oncol 2013;131:714–9.
244 Cusimano MC, Vicus D, Pulman K, etal. Assessment of sentinel
lymph node biopsy vs lymphadenectomy for intermediate- and
high- grade endometrial cancer staging. JAMA Surg 2020.
doi:10.1001/jamasurg.2020.5060. [Epub ahead of print: 11 Nov
2020].
245 Rozenholc A, Samouelian V, Warkus T, etal. Green versus blue:
randomized controlled trial comparing indocyanine green with
methylene blue for sentinel lymph node detection in endometrial
cancer. Gynecol Oncol 2019;153:500–4.
246 Kang S, Yoo HJ, Hwang JH, etal. Sentinel lymph node biopsy in
endometrial cancer: meta- analysis of 26 studies. Gynecol Oncol
2011;123:522–7.
247 Frumovitz M, Plante M, Lee PS, etal. Near- infrared uorescence
for detection of sentinel lymph nodes in women with cervical and
uterine cancers (FILM): a randomised, phase 3, multicentre, non-
inferiority trial. Lancet Oncol 2018;19:1394–403.
248 Ignatov A, Lebius C, Ignatov T, etal. Lymph node micrometastases
and outcome of endometrial cancer. Gynecol Oncol
2019;154:475–9.
249 Kitchener H, Swart AMC, etal, ASTEC Study Group. Efcacy of
systematic pelvic lymphadenectomy in endometrial cancer (MRC
ASTEC trial): a randomised study. Lancet 2009;373:125–36.
250 Benedetti Panici P, Basile S, Maneschi F, etal. Systematic pelvic
lymphadenectomy vs. no lymphadenectomy in early- stage
endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst
2008;100:1707–16.
251 Gu H, Li J, Gu Y, etal. Survival impact of ovarian preservation on
women with early- stage endometrial cancer: a systematic review
and meta- analysis. Int J Gynecol Cancer 2017;27:77–84.
252 Lyu T, Guo L, Chen X, etal. Ovarian preservation for
premenopausal women with early- stage endometrial cancer: a
Chinese retrospective study. J Int Med Res 2019;47:2492–8.
253 Lau H- Y, Chen M- Y, Ke Y- M, etal. Outcome of ovarian preservation
during surgical treatment for endometrial cancer: a Taiwanese
Gynecologic Oncology Group study. Taiwan J Obstet Gynecol
2015;54:532–6.
254 Kinjyo Y, Kudaka W, Ooyama T, etal. Ovarian preservation in young
women with endometrial cancer of endometrioid histology. Acta
Obstet Gynecol Scand 2015;94:430–4.
255 Anggraeni TD, Al Fattah AN, Surya R. Prophylactic salpingectomy
and ovarian cancer: an evidence- based analysis. South Asian J
Cancer 2018;7:42–5.
256 Peccatori FA, Mangili G, Bergamini A, etal. Fertility preservation
in women harboring deleterious BRCA mutations: ready for prime
time? Hum Reprod 2018;33:181–7.
257 Liu T, Tu H, Li Y, etal. Impact of radical hysterectomy versus simple
hysterectomy on survival of patients with stage 2 endometrial
cancer: a meta- analysis. Ann Surg Oncol 2019;26:2933–42.
258 van der Steen- Banasik E, Christiaens M, Shash E, etal. Systemic
review: radiation therapy alone in medical non- operable
endometrial carcinoma. Eur J Cancer 2016;65:172–81.
259 Dutta SW, Triletti DM, Grover S, etal. Management of elderly
patients with early- stage medically inoperable endometrial cancer:
systematic review and National Cancer Database analysis.
Brachytherapy 2017;16:526–33.
260 Acharya S, Perkins SM, DeWees T, etal. Brachytherapy is
associated with improved survival in inoperable stage I endometrial
adenocarcinoma: a population- based analysis. Int J Radiat Oncol
Biol Phys 2015;93:649–57.
261 Schwarz JK, Beriwal S, Esthappan J, etal. Consensus statement
for brachytherapy for the treatment of medically inoperable
endometrial cancer. Brachytherapy 2015;14:587–99.
262 Gill BS, Kim H, Houser C, etal. Image- based three- dimensional
conformal brachytherapy for medically inoperable endometrial
carcinoma. Brachytherapy 2014;13:542–7.
263 Yang B, Xu Y, Zhu Q, etal. Treatment efciency of comprehensive
hysteroscopic evaluation and lesion resection combined
with progestin therapy in young women with endometrial
atypical hyperplasia and endometrial cancer. Gynecol Oncol
2019;153:55–62.
264 Chae SH, Shim S- H, Lee SJ, etal. Pregnancy and oncologic
outcomes after fertility- sparing management for early stage
endometrioid endometrial cancer. Int J Gynecol Cancer
2019;29:77–85.
265 Giampaolino P, Di Spiezio Sardo A, Mollo A, etal. Hysteroscopic
endometrial focal resection followed by levonorgestrel intrauterine
device insertion as a fertility- sparing treatment of atypical
endometrial hyperplasia and early endometrial cancer: a
retrospective study. J Minim Invasive Gynecol 2019;26:648–56.
266 Tamauchi S, Kajiyama H, Utsumi F, etal. Efcacy of
medroxyprogesterone acetate treatment and retreatment for
atypical endometrial hyperplasia and endometrial cancer. J Obstet
Gynaecol Res 2018;44:151–6.
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2020-002230 on 18 December 2020. Downloaded from

36
ConcinN, etal. Int J Gynecol Cancer 2021;31:12–39. doi:10.1136/ijgc-2020-002230
Joint statement
267 Kim SR, van der Zanden C, Ikiz H, etal. Fertility- sparing
management using progestin for young women with endometrial
cancer from a population- based study. J Obstet Gynaecol Can
2018;40:328–33.
268 Yamagami W, Susumu N, Makabe T, etal. Is repeated high- dose
medroxyprogesterone acetate (MPA) therapy permissible for
patients with early stage endometrial cancer or atypical endometrial
hyperplasia who desire preserving fertility? J Gynecol Oncol
2018;29:e21.
269 Fan Z, Li H, Hu R, etal. Fertility- preserving treatment in
young women with grade 1 presumed stage Ia endometrial
adenocarcinoma: a meta- analysis. Int J Gynecol Cancer
2018;28:385–93.
270 Hwang JY, Kim DH, Bae HS, etal. Combined oral
medroxyprogesterone/levonorgestrel- intrauterine system treatment
for women with grade 2 stage Ia endometrial cancer. Int J Gynecol
Cancer 2017;27:738–42.
271 Di Spiezio Sardo A, De Angelis MC, Della Corte L, etal. Should
endometrial biopsy under direct hysteroscopic visualization using
the GRASP technique become the new gold standard for the
preoperative evaluation of the patient with endometrial cancer?
Gynecol Oncol 2020;158:347–53.
272 Lago V, Martín B, Ballesteros E, etal. Tumor grade correlation
between preoperative biopsy and nal surgical specimen in
endometrial cancer: the use of different diagnostic methods
and analysis of associated factors. Int J Gynecol Cancer
2018;28:1258–63.
273 Larish A, Kumar A, Weaver A, etal. Impact of hysteroscopy on
course of disease in high- risk endometrial carcinoma. Int J Gynecol
Cancer 2020;30:1513–9.
274 Education and Practical Standards Committee, European
Federation of Societies for Ultrasound in Medicine and Biology.
Minimum training recommendations for the practice of medical
ultrasound. Ultraschall Med 2006;27:79–105.
275 Kitson S, Ryan N, MacKintosh ML, etal. Interventions for weight
reduction in obesity to improve survival in women with endometrial
cancer. Cochrane Database Syst Rev 2018;2.
276 Raffone A, Travaglino A, Saccone G, etal. Diabetes mellitus
and responsiveness of endometrial hyperplasia and early
endometrial cancer to conservative treatment. Gynecol Endocrinol
2019;35:932–7.
277 Chu D, Wu J, Wang K, etal. Effect of metformin use on the risk and
prognosis of endometrial cancer: a systematic review and meta-
analysis. BMC Cancer 2018;18:438.
278 Greenwald ZR, Huang LN, Wissing MD, etal. Does hormonal
therapy for fertility preservation affect the survival of young women
with early- stage endometrial cancer? Cancer 2017;123:1545–54.
279 Wei J, Zhang W, Feng L, etal. Comparison of fertility- sparing
treatments in patients with early endometrial cancer and atypical
complex hyperplasia. Medicine 2017;96:e8034.
280 Zhang Q, Qi G, Kanis MJ, etal. Comparison among fertility-
sparing therapies for well differentiated early- stage endometrial
carcinoma and complex atypical hyperplasia. Oncotarget
2017;8:57642–53.
281 Zapardiel I, Cruz M, Diestro MD, etal. Assisted reproductive
techniques after fertility- sparing treatments in gynaecological
cancers. Hum Reprod Update 2016;22:281–305.
282 Marton I, Vranes HS, Sparac V, etal. Two cases of successful
pregnancies after hysteroscopic removal of endometrioid
adenocarcinoma grade I, stage Ia, in young women with Lynch
syndrome. J Turkish German Gynecol Assoc 2014;15:63–6.
283 Casadio P, Guasina F, Talamo MR, etal. Conservative
hysteroscopic treatment of stage I well differentiated endometrial
cancer in patients with high surgical risk: a pilot study. J Gynecol
Oncol 2019;30:e62.
284 Yang H- C, Liu J- C, Liu F- S. Fertility- preserving treatment of stage
Ia, well- differentiated endometrial carcinoma in young women
with hysteroscopic resection and high- dose progesterone therapy.
Taiwan J Obstet Gynecol 2019;58:90–3.
285 Arendas K, Aldossary M, Cipolla A, etal. Hysteroscopic resection
in the management of early- stage endometrial cancer: report of
2 cases and review of the literature. J Minim Invasive Gynecol
2015;22:34–9.
286 Casadio P, Guasina F, Paradisi R, etal. Fertility‐sparing treatment
of endometrial cancer with initial inltration of myometrium by
resectoscopic surgery: a pilot study. Oncologist 2018;23:478–80.
287 Leone Roberti Maggiore U, Martinelli F, Dondi G, etal. Efcacy
and fertility outcomes of levonorgestrel- releasing intra- uterine
system treatment for patients with atypical complex hyperplasia
or endometrial cancer: a retrospective study. J Gynecol Oncol
2019;30:e57.
288 Pal N, Broaddus RR, Urbauer DL, etal. Treatment of low- risk
endometrial cancer and complex atypical hyperplasia with the
levonorgestrel- releasing intrauterine device. Obstet Gynecol
2018;131:109–16.
289 Sletten ET, Arnes M, Vereide AB, etal. Low- dose LNG- IUS as
therapy for endometrial hyperplasia. A prospective cohort pilot
study. Anticancer Res 2018;38:2883–9.
290 Ørbo A, Vereide AB, Arnes M, etal. Levonorgestrel‐impregnated
intrauterine device as treatment for endometrial hyperplasia: a
national multicentre randomised trial. BJOG: Int J Obstet Gynaecol
2014;121:477–86.
291 Luo L, Luo B, Zheng Y, etal. Oral and intrauterine progestogens
for atypical endometrial hyperplasia. Cochrane Database Syst Rev
2018;12.
292 Marnach ML, Butler KA, Henry MR, etal. Oral Progestogens Versus
Levonorgestrel- Releasing Intrauterine System for Treatment of
Endometrial Intraepithelial. J Womens Health 2017;26:368–73.
293 Kim MK, Seong SJ, Kang S- B, etal. Six months response rate of
combined oral medroxyprogesterone/levonorgestrel- intrauterine
system for early- stage endometrial cancer in young women: a
Korean Gynecologic- Oncology Group study. J Gynecol Oncol
2019;30:e47.
294 Tock S, Jadoul P, Squifet J- L, etal. Fertility sparing treatment in
patients with early stage endometrial cancer, using a combination
of surgery and GnRH agonist: a monocentric retrospective study
and review of the literature. Front Med 2018;5.
295 Jadoul P, Donnez J. Conservative treatment may be benecial for
young women with atypical endometrial hyperplasia or endometrial
adenocarcinoma. Fertil Steril 2003;80:1315–24.
296 Stewart CJR, Crum CP, McCluggage WG, etal. Guidelines to aid
in the distinction of endometrial and endocervical carcinomas,
and the distinction of independent primary carcinomas of the
endometrium and adnexa from metastatic spread between these
and other sites. Int J Gynecol Pathol 2019;38 Suppl 1:S75–92.
297 Connell PP, Rotmensch J, Waggoner S, etal. The signicance of
adnexal involvement in endometrial carcinoma. Gynecol Oncol
1999;74:74–9.
298 Soliman PT, Slomovitz BM, Broaddus RR, etal. Synchronous
primary cancers of the endometrium and ovary: a single institution
review of 84 cases. Gynecol Oncol 2004;94:456–62.
299 Schultheis AM, Ng CKY, De Filippo MR, etal. Massively parallel
sequencing- based clonality analysis of synchronous endometrioid
endometrial and ovarian carcinomas. J Natl Cancer Inst
2016;108:djv427.
300 Anglesio MS, Wang YK, Maassen M, etal. Synchronous
endometrial and ovarian carcinomas: evidence of clonality. J Natl
Cancer Inst 2016;108:djv428.
301 Turashvili G, Gómez- Hidalgo NR, Flynn J, etal. Risk- based
stratication of carcinomas concurrently involving the endometrium
and ovary. Gynecol Oncol 2019;152:38–45.
302 Keys HM, Roberts JA, Brunetto VL, etal. A phase III trial of surgery
with or without adjunctive external pelvic radiation therapy in
intermediate risk endometrial adenocarcinoma: a Gynecologic
Oncology Group study. Gynecol Oncol 2004;92:744–51.
303 Creutzberg CL, van Putten WLJ, Koper PCM, etal. Surgery and
postoperative radiotherapy versus surgery alone for patients with
stage-1 endometrial carcinoma: multicentre randomised trial. The
Lancet 2000;355:1404–11.
304 Aalders J, Abeler V, Kolstad P, etal. Postoperative external
irradiation and prognostic parameters in stage I endometrial
carcinoma: clinical and histopathologic study of 540 patients.
Obstet Gynecol 1980;56:419–27.
305 Blake P, Swart AM, etal, ASTEC/EN.5 Study Group. Adjuvant
external beam radiotherapy in the treatment of endometrial
cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials):
pooled trial results, systematic review, and meta- analysis. Lancet
2009;373:137–46.
306 Barney BM, Petersen IA, Mariani A, etal. The role of vaginal
brachytherapy in the treatment of surgical stage I papillary serous
or clear cell endometrial cancer. Int J Radiat Oncol Biol Phys
2013;85:109–15.
307 Wortman BG, Creutzberg CL, Putter H, etal. Ten- year results of the
PORTEC-2 trial for high- intermediate risk endometrial carcinoma:
improving patient selection for adjuvant therapy. Br J Cancer
2018;119:1067–74.
308 Nout RA, Smit V, Putter H, etal. Vaginal brachytherapy versus
pelvic external beam radiotherapy for patients with endometrial
cancer of high- intermediate risk (PORTEC-2): an open- label, non-
inferiority, randomised trial. The Lancet 2010;375:816–23.
309 Sunil RA, Bhavsar D, Shruthi MN, etal. Combined external
beam radiotherapy and vaginal brachytherapy versus vaginal
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2020-002230 on 18 December 2020. Downloaded from

37
ConcinN, etal. Int J Gynecol Cancer 2021;31:12–39. doi:10.1136/ijgc-2020-002230
Joint statement
brachytherapy in stage I, intermediate- and high- risk cases
of endometrium carcinoma. J Contemp Brachytherapy
2018;10:105–14.
310 Cham S, Huang Y, Tergas AI, etal. Utility of radiation therapy for
early- stage uterine papillary serous carcinoma. Gynecol Oncol
2017;145:269–76.
311 Shinde A, Li R, Amini A, etal. Improved survival with adjuvant
brachytherapy in stage Ia endometrial cancer of unfavorable
histology. Gynecol Oncol 2018;151:82–90.
312 Qu XM, Velker VM, Leung E, etal. The role of adjuvant therapy in
stage Ia serous and clear cell uterine cancer: a multi- institutional
pooled analysis. Gynecol Oncol 2018;149:283–90.
313 Donovan E, Reade CJ, Eiriksson LR, etal. Outcomes of
adjuvant therapy for stage Ia serous endometrial cancer. Cureus
2018;10:e3387.
314 Sorbe B, Horvath G, Andersson H, etal. External pelvic
and vaginal irradiation versus vaginal irradiation alone as
postoperative therapy in medium- risk endometrial carcinoma—a
prospective randomized study. Int J Radiat Oncol Biol Phys
2012;82:1249–55.
315 Ørtoft G, Hansen ES, Bertelsen K. Omitting adjuvant radiotherapy
in endometrial cancer increases the rate of locoregional
recurrences but has no effect on long- term survival: the
Danish endometrial cancer study. Int J Gynecol Cancer
2013;23:1429–37.
316 Randall ME, Filiaci V, McMeekin DS, etal. Phase III trial: adjuvant
pelvic radiation therapy versus vaginal brachytherapy plus
paclitaxel/carboplatin in high- intermediate and high- risk early- stage
endometrial cancer. JCO 2019;37:1810–8.
317 Maggi R, Lissoni A, Spina F, etal. Adjuvant chemotherapy vs
radiotherapy in high- risk endometrial carcinoma: results of a
randomised trial. Br J Cancer 2006;95:266–71.
318 Susumu N, Sagae S, Udagawa Y, etal. Randomized phase III
trial of pelvic radiotherapy versus cisplatin- based combined
chemotherapy in patients with intermediate- and high- risk
endometrial cancer: a Japanese Gynecologic Oncology Group
study. Gynecol Oncol 2008;108:226–33.
319 Hogberg T, Signorelli M, de Oliveira CF, etal. Sequential adjuvant
chemotherapy and radiotherapy in endometrial cancer–Results
from two randomised studies. Eur J Cancer 2010;46:2422–31.
320 de Boer SM, Powell ME, Mileshkin L, etal. Adjuvant
chemoradiotherapy versus radiotherapy alone for women with
high- risk endometrial cancer (PORTEC-3): nal results of an
international, open- label, multicentre, randomised, phase 3 trial.
Lancet Oncol 2018;19:295–309.
321 León- Castillo A, de Boer SM, Powell ME, etal. Molecular
classication of the PORTEC-3 trial for high- risk endometrial
cancer: impact on prognosis and benet from adjuvant therapy.
JCO 2020;38:3388–97.
322 Randall M, Filiaci V, McMeekin D, etal. A phase 3 trial of pelvic
radiation therapy versus vaginal cuff brachytherapy followed by
paclitaxel/carboplatin chemotherapy in patients with high- risk,
early- stage endometrial cancer: a Gynecology Oncology Group
study. Int J Radiat Oncol Biol Phys 2017;99:1313.
323 de Boer SM, Powell ME, Mileshkin L, etal. Adjuvant
chemoradiotherapy versus radiotherapy alone in women with high-
risk endometrial cancer (PORTEC-3): patterns of recurrence and
post- hoc survival analysis of a randomised phase 3 trial. Lancet
Oncol 2019;20:1273–85.
324 Matei D, Filiaci V, Randall ME, etal. Adjuvant chemotherapy plus
radiation for locally advanced endometrial cancer. N Engl J Med
Overseas Ed 2019;380:2317–26.
325 Randall ME, Filiaci V, McMeekin DS, etal. Phase III trial: adjuvant
pelvic radiation therapy versus vaginal brachytherapy plus
paclitaxel/carboplatin in high- intermediate and high- risk early stage
endometrial cancer. J Clin Oncol 2019;37:1810–8.
326 Albeesh R, Turgeon G- A, Aleri J, etal. Adjuvant therapy in stage
III endometrial cancer conned to the pelvis. Gynecol Oncol
2019;152:26–30.
327 Onal C, Yildirim BA, Sari SY, etal. Treatment outcomes of
endometrial cancer patients with paraaortic lymph node
metastasis: a multi- institutional analysis. Int J Gynecol Cancer
2019;29:94–101.
328 Scharl S, Papathemelis T, Kronberger K, etal. Does post- operative
radiochemotherapy improve survival in high- grade endometrial
cancer patients? Results of a population- based cohort analysis of a
cancer registry. Arch Gynecol Obstet 2018;297:1245–53.
329 Bonadio RR da CC, Azevedo R, Harada G. Adjuvant carboplatin
and paclitaxel chemotherapy followed by radiotherapy in high-
risk endometrial cancer: a retrospective analysis. J Glob Oncol
2018;4:1–8.
330 Chapman BV, Swanick CW, Ning MS, etal. Adjuvant combined-
modality therapy for stage IIIC endometrioid and non- endometrioid
endometrial cancer. Gynecol Oncol 2019;154:22–8.
331 Binder PS, Kuroki LM, Zhao P, etal. Benet of combination
chemotherapy and radiation stratied by grade of stage IIIC
endometrial cancer. Gynecol Oncol 2017;147:309–14.
332 Lee JK, Mahan M, Hanna RK, etal. Survival outcomes and patterns
of failure in women with stage IIIC2 endometrial carcinoma. Eur J
Obstet Gynecol Reprod Biol 2017;216:192–7.
333 Signorelli M, Lissoni AA, De Ponti E, etal. Adjuvant sequential
chemo and radiotherapy improves the oncological outcome in high
risk endometrial cancer. J Gynecol Oncol 2015;26:284–92.
334 Bogani G, Cromi A, Serati M, etal. Chemotherapy reduces
para- aortic node recurrences in endometrial cancer with positive
pelvic and unknown para- aortic nodes. Int J Gynecol Cancer
2015;25:263–8.
335 Lee LJ, Bu P, Feltmate C, etal. Adjuvant chemotherapy with
external beam radiation therapy for high- grade, node- positive
endometrial cancer. Int J Gynecol Cancer 2014;24:1441–8.
336 Bakkum- Gamez JN, Mariani A, Dowdy SC, etal. Efcacy of
contemporary chemotherapy in stage IIIC endometrial cancer: a
histologic dichotomy. Gynecol Oncol 2014;132:578–84.
337 Xiang M, English DP, Kidd EA. National patterns of care and
cancer- specic outcomes of adjuvant treatment in patients with
serous and clear cell endometrial carcinoma. Gynecol Oncol
2019;152:599–604.
338 Holloway CL, Alexander C, Walter C, etal. Stage IIIC endometrial
cancer: relapse and survival outcomes in women treated with
pelvic or extended eld para- aortic nodal radiation therapy. Am J
Clin Oncol 2017;40:458–63.
339 Chen J- R, Chang T- C, Fu H- C, etal. Outcomes of patients with
surgically and pathologically staged IIIA- IVB pure endometrioid-
type endometrial cancer: a Taiwanese Gynecology Oncology Group
(TGOG-2005) retrospective cohort study (a STROBE- compliant
article). Medicine 2016;95:e3330.
340 Fleming ND, Soliman PT, Westin SN, etal. Impact of lymph
node ratio and adjuvant therapy in node- positive endometrioid
endometrial cancer. Int J Gynecol Cancer 2015;25:1437–44.
341 Boothe D, Orton A, Odei B, etal. Chemoradiation versus
chemotherapy or radiation alone in stage III endometrial cancer:
patterns of care and impact on overall survival. Gynecol Oncol
2016;141:421–7.
342 Boothe D, Orton A, Odei B, etal. Corrigendum to “Chemoradiation
versus chemotherapy or radiation alone in stage III endometrial
cancer: Patterns of care and impact on overall survival” [Gynecol.
Oncol. 141 (2016) 421–427]. Gynecol Oncol 2016;143:690–1.
343 Wong AT, Rineer J, Lee Y- C, etal. Utilization of adjuvant therapies
and their impact on survival for women with stage IIIC endometrial
adenocarcinoma. Gynecol Oncol 2016;142:514–9.
344 Lin JF, Muñiz K, Sukumvanich P, etal. Survival advantage
associated with multimodal therapy in women with node- positive
(stage- IIIC) uterine papillary serous carcinoma: a National Cancer
Database study. Int J Obstet Gynaecol 2016;123:1846–52.
345 Barlin JN, Puri I, Bristow RE. Cytoreductive surgery for advanced
or recurrent endometrial cancer: a meta- analysis. Gynecol Oncol
2010;118:14–18.
346 Rajkumar S, Nath R, Lane G, etal. Advanced stage (IIIC/IV)
endometrial cancer: role of cytoreduction and determinants of
survival. Eur J Obstet Gynecol Reprod Biol 2019;234:26–31.
347 Solmaz U, Mat E, Dereli ML, etal. Stage- III and -IV endometrial
cancer: a single oncology centre review of 104 cases. J Obstet
Gynaecol 2016;36:81–6.
348 Cirik DA, Karalok A, Ureyen I, etal. Stage IVb endometrial
cancer conned to the abdomen: is chemotherapy superior to
radiotherapy? Eur J Gynaecol Oncol 2016;37:226–31.
349 Schmidt A- M, Imesch P, Fink D, etal. Pelvic exenterations for
advanced and recurrent endometrial cancer: clinical outcomes of
40 patients. Int J Gynecol Cancer 2016;26:716–21.
350 Vitale SG, Valenti G, Gulino FA, etal. Surgical treatment of high
stage endometrial cancer: current perspectives. Updates Surg
2016;68:149–54.
351 Tangjitgamol S, Kittisiam T, Sriraumpuch J. Impact of metastatic
lymph node to total lymph node ratio on survival of endometrial
cancer patients. Gynecol Obstet Invest 2019;84:463–71.
352 Yoon MS, Park W, Huh SJ, etal. Impact of paraaortic
lymphadenectomy for endometrial cancer with positive pelvic
lymph nodes: a Korean Radiation Oncology Group study (KROG
13-17). Eur J Surg Oncol 2016;42:1497–505.
353 Bogani G, Ditto A, Maggiore ULR, etal. Neoadjuvant chemotherapy
followed by interval debulking surgery for unresectable stage IVb
serous endometrial cancer. Tumori 2019;105:92–7.
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2020-002230 on 18 December 2020. Downloaded from

38
ConcinN, etal. Int J Gynecol Cancer 2021;31:12–39. doi:10.1136/ijgc-2020-002230
Joint statement
354 Boisen MM, Vargo JA, Beriwal S, etal. Surgical outcomes of
patients undergoing extrafascial hysterectomy after neoadjuvant
radiotherapy with or without chemotherapy for locally advanced
endometrial cancer clinically extending to the cervix or parametria.
Int J Gynecol Cancer 2017;27:1149–54.
355 De Lange NM, Ezendam NPM, Kwon JS, etal. Neoadjuvant
chemotherapy followed by surgery for advanced- stage endometrial
cancer. Curr Oncol 2019;26:e226–32.
356 Iheagwara UK, Vargo JA, Chen KS, etal. Neoadjuvant
chemoradiation therapy followed by extrafascial hysterectomy in
locally advanced type II endometrial cancer clinically extending to
cervix. Pract Radiat Oncol 2019;9:248–56.
357 Khouri OR, Frey MK, Musa F, etal. Neoadjuvant chemotherapy in
patients with advanced endometrial cancer. Cancer Chemother
Pharmacol 2019;84:281–5.
358 Palisoul M, Mutch DG. The clinical management of inoperable
endometrial carcinoma. Expert Rev Anticancer Ther
2016;16:515–21.
359 Rabinovich A. Neo- adjuvant chemotherapy for advanced stage
endometrial carcinoma: a glimmer of hope in select patients. Arch
Gynecol Obstet 2016;293:47–53.
360 Vandenput I, Van Calster B, Capoen A, etal. Neoadjuvant
chemotherapy followed by interval debulking surgery in patients
with serous endometrial cancer with transperitoneal spread (stage
IV): a new preferred treatment? Br J Cancer 2009;101:244–9.
361 Conway JL, Lukovic J, Laframboise S, etal. Brachy- ing
unresectable endometrial cancers with magnetic resonance
guidance. Cureus 2018;10:e2274.
362 Townamchai K, Poorvu PD, Damato AL, etal. Radiation dose
escalation using intensity modulated radiation therapy for gross
unresected node- positive endometrial cancer. Pract Radiat Oncol
2014;4:90–8.
363 Francis SR, Ager BJ, Do OA, etal. Recurrent early stage
endometrial cancer: patterns of recurrence and results of salvage
therapy. Gynecol Oncol 2019;154:38–44.
364 Hardarson HA, Heidemann LN, dePont Christensen R, etal. Vaginal
vault recurrences of endometrial cancer in non- irradiated patients
— radiotherapy or surgery. Gynecol Oncol Rep 2015;11:26–30.
365 Shikama A, Minaguchi T, Takao W, etal. Predictors of favorable
survival after secondary cytoreductive surgery for recurrent
endometrial cancer. Int J Clin Oncol 2019;24:1256–63.
366 Turan T, Tasci T, Karalok A, etal. Salvage cytoreductive
surgery for recurrent endometrial cancer. Int J Gynecol Cancer
2015;25:1623–32.
367 Ren Y, Shan B, Shi D, etal. Salvage cytoreductive surgery for
patients with recurrent endometrial cancer: a retrospective study.
BMC Cancer 2014;14:135.
368 Papadia A, Bellati F, Ditto A, etal. Surgical treatment of recurrent
endometrial cancer: time for a paradigm shift. Ann Surg Oncol
2015;22:4204–10.
369 Domenici L, Nixon K, Sorbi F, etal. Surgery for recurrent uterine
cancer: surgical outcomes and implications for survival—a case
series. Int J Gynecol Cancer 2017;27:759–67.
370 Baek S, Isohashi F, Yamaguchi H, etal. Salvage high- dose- rate
brachytherapy for isolated vaginal recurrence of endometrial
cancer. Brachytherapy 2016;15:812–6.
371 Chapman CH, Maghsoudi K, Littell RD, etal. Salvage high- dose-
rate brachytherapy and external beam radiotherapy for isolated
vaginal recurrences of endometrial cancer with no prior adjuvant
therapy. Brachytherapy 2017;16:1152–8.
372 Fokdal L, Ørtoft G, Hansen ES, etal. Toward four- dimensional
image- guided adaptive brachytherapy in locally recurrent
endometrial cancer. Brachytherapy 2014;13:554–61.
373 Ho JC, Allen PK, Jhingran A, etal. Management of nodal
recurrences of endometrial cancer with IMRT. Gynecol Oncol
2015;139:40–6.
374 Huang K, D'Souza D, Patil N, etal. High- dose- rate interstitial
brachytherapy for the treatment of high- volume locally recurrent
endometrial carcinoma. Brachytherapy 2016;15:543–8.
375 Kamran SC, Manuel MM, Catalano P, etal. MR- versus CT- based
high- dose- rate interstitial brachytherapy for vaginal recurrence of
endometrial cancer. Brachytherapy 2017;16:1159–68.
376 Sekii S, Murakami N, Kato T, etal. Outcomes of salvage high- dose-
rate brachytherapy with or without external beam radiotherapy
for isolated vaginal recurrence of endometrial cancer. J Contemp
Brachytherapy 2017;3:209–15.
377 Vargo JA, Kim H, Houser CJ, etal. Denitive salvage for vaginal
recurrence of endometrial cancer: the impact of modern intensity-
modulated- radiotherapy with image- based HDR brachytherapy and
the interplay of the PORTEC 1 risk stratication. Radiother Oncol
2014;113:126–31.
378 Viswanathan AN, Lee H, Berkowitz R, etal. A prospective feasibility
study of radiation and concurrent bevacizumab for recurrent
endometrial cancer. Gynecol Oncol 2014;132:55–60.
379 Yanazume S, Arimura T, Kobayashi H, etal. Potential proton beam
therapy for recurrent endometrial cancer in the vagina. J Obstet
Gynaecol Res 2015;41:813–6.
380 Chiantera V, Rossi M, De Iaco P, etal. Pelvic exenteration for
recurrent endometrial adenocarcinoma: a retrospective multi-
institutional study about 21 patients. Int J Gynecol Cancer
2014;24:880–4.
381 Margolis B, Kim SW, Chi DS. Long- term survival after anterior
pelvic exenteration and total vaginectomy for recurrent endometrial
carcinoma with metastatic inguinal nodes at the time of surgery.
Gynecol Oncol Rep 2017;19:39–41.
382 Ling DC, Vargo JA, Glaser SM, etal. Outcomes after denitive
re- irradiation with 3D brachytherapy with or without external beam
radiation therapy for vaginal recurrence of endometrial cancer.
Gynecol Oncol 2019;152:581–6.
383 Mabuchi S, Takahashi R, Isohashi F, etal. Reirradiation using high-
dose- rate interstitial brachytherapy for locally recurrent cervical
cancer: a single institutional experience. Int J Gynecol Cancer
2014;24:141–8.
384 Arians N, Foerster R, Rom J, etal. Outcome of patients with local
recurrent gynecologic malignancies after resection combined with
intraoperative electron radiation therapy (IOERT). Radiat Oncol
2016;11.
385 Feddock J, Cheek D, Steber C, etal. Reirradiation using permanent
interstitial brachytherapy: a potentially durable technique for
salvaging recurrent pelvic malignancies. Int J Radiat Oncol Biol
Phys 2017;99:1225–33.
386 Wooten CE, Randall M, Edwards J, etal. Implementation and
early clinical results utilizing Cs-131 permanent interstitial
implants for gynecologic malignancies. Gynecol Oncol
2014;133:268–73.
387 Widder J, Lodeweges J. Synchronous or metachronous
oligometastases. J Thorac Oncol 2017;12:e191–2.
388 Xu L, Burke AP. Pulmonary oligometastases: histological features
and difculties in determining site of origin. Int J Surg Pathol
2012;20:577–88.
389 Kaneda H, Saito Y. Oligometastases: dened by prognosis and
evaluated by cure. Cancer Treat Commun 2015;3:1–6.
390 Kunos CA, Brindle J, Waggoner S, etal. Phase II clinical trial of
robotic stereotactic body radiosurgery for metastatic gynecologic
malignancies. Front Oncol 2012;2:181.
391 Lodeweges JE, Klinkenberg TJ, Ubbels JF, etal. Long- term
outcome of surgery or stereotactic radiotherapy for lung
oligometastases. J Thorac Oncol 2017;12:1442–5.
392 Palma DA, Olson R, Harrow S, etal. Stereotactic ablative
radiotherapy versus standard of care palliative treatment in patients
with oligometastatic cancers (SABR- COMET): a randomised, phase
2, open- label trial. Lancet 2019;393:2051–8.
393 Loveman E, Jones J, Clegg AJ, etal. The clinical effectiveness and
cost- effectiveness of ablative therapies in the management of liver
metastases: systematic review and economic evaluation. Health
Technol Assess 2014;18:1–283.
394 van Weelden WJ, Massuger LFAG, etal, ENITEC. Anti- estrogen
treatment in endometrial cancer: a systematic review. Front Oncol
2019;9:359.
395 Ethier J- L, Desautels DN, Amir E, etal. Is hormonal therapy
effective in advanced endometrial cancer? A systematic review and
meta- analysis. Gynecol Oncol 2017;147:158–66.
396 Mileshkin L, Edmondson R, O'Connell RL, etal. Phase 2 study of
anastrozole in recurrent estrogen (ER)/progesterone (PR) positive
endometrial cancer: The PARAGON trial – ANZGOG 0903. Gynecol
Oncol 2019;154:29–37.
397 Slomovitz BM, Jiang Y, Yates MS, etal. Phase II study of
everolimus and letrozole in patients with recurrent endometrial
carcinoma. JCO 2015;33:930–6.
398 Miller DFV, Filiaci V, Fleming G, etal. Randomized phase III
non inferiority trial of rst line chemotherapy for metastatic or
recurrent endometrial carcinoma: a gynecologic oncology group
study. [SGO abstract Late- Breaking Abstract 1]. Gynecol Oncol
2012;125S:771–3.
399 Rubinstein M, Halpenny D, Makker V, etal. Retreatment with
carboplatin and paclitaxel for recurrent endometrial cancer: a
retrospective study of the Memorial Sloan Kettering Cancer Center
experience. Gynecol Oncol Rep 2019;28:120–3.
400 Marabelle A, Le DT, Ascierto PA, etal. Efcacy of pembrolizumab in
patients with noncolorectal high microsatellite instability/mismatch
repair- decient cancer: results from the phase II KEYNOTE-158
study. J Clin Oncol 2020;38:1–10.
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2020-002230 on 18 December 2020. Downloaded from

39
ConcinN, etal. Int J Gynecol Cancer 2021;31:12–39. doi:10.1136/ijgc-2020-002230
Joint statement
401 Mittica G, Ghisoni E, Giannone G, etal. Checkpoint inhibitors
in endometrial cancer: preclinical rationale and clinical activity.
Oncotarget 2017;8:90532–44.
402 Makker V, Taylor MH, Aghajanian C, etal. Lenvatinib plus
pembrolizumab in patients with advanced endometrial cancer. J
Clin Oncol 2020;38:2981–92.
403 Makker V, Rasco D, Vogelzang NJ, etal. Lenvatinib plus
pembrolizumab in patients with advanced endometrial cancer: an
interim analysis of a multicentre, open- label, single- arm, phase 2
trial. Lancet Oncol 2019;20:711–8.
404 Fader AN, Roque DM, Siegel E, etal. Randomized phase II trial of
carboplatin- paclitaxel versus carboplatin- paclitaxel- trastuzumab
in uterine serous carcinomas that overexpress human epidermal
growth factor receptor 2/neu. J Clin Oncol 2018;36:2044–51.
405 Roncolato F, Lindemann K, Willson ML, etal. Pi3K/Akt/mTOR
inhibitors for advanced or recurrent endometrial cancer. Cochrane
Database Syst Rev 2019;10.
406 Coleman RL, Sill MW, Thaker PH, etal. A phase II evaluation
of selumetinib (AZD6244, ARRY-142886), a selective MEK-1/2
inhibitor in the treatment of recurrent or persistent endometrial
cancer: an NRG Oncology/Gynecologic Oncology Group study.
Gynecol Oncol 2015;138:30–5.
407 Bender D, Sill MW, Lankes HA, etal. A phase II evaluation of
cediranib in the treatment of recurrent or persistent endometrial
cancer: an NRG Oncology/Gynecologic Oncology Group study.
Gynecol Oncol 2015;138:507–12.
408 Dizon DS, Sill MW, Schilder JM, etal. A phase II evaluation of
nintedanib (BIBF-1120) in the treatment of recurrent or persistent
endometrial cancer: an NRG Oncology/Gynecologic Oncology
Group study. Gynecol Oncol 2014;135:441–5.
409 Castonguay V, Lheureux S, Welch S, etal. A phase II trial of
sunitinib in women with metastatic or recurrent endometrial
carcinoma: a study of the Princess Margaret, Chicago and
California consortia. Gynecol Oncol 2014;134:274–80.
410 Powell MA, Sill MW, Goodfellow PJ, etal. A phase II trial of
brivanib in recurrent or persistent endometrial cancer: an NRG
Oncology/Gynecologic Oncology Group study. Gynecol Oncol
2014;135:38–43.
411 Oza AM, Elit L, Tsao M- S, etal. Phase II study of temsirolimus in
women with recurrent or metastatic endometrial cancer: a trial of
the NCIC Clinical Trials Group. J Clin Oncol 2011;29:3278–85.
412 Slomovitz BM, Lu KH, Johnston T, etal. A phase 2 study of the oral
mammalian target of rapamycin inhibitor, everolimus, in patients
with recurrent endometrial carcinoma. Cancer 2010;116:5415–9.
413 Emons G, Kurzeder C, Schmalfeldt B, etal. Temsirolimus in women
with platinum- refractory/resistant ovarian cancer or advanced/
recurrent endometrial carcinoma. A phase II study of the AGO-
study group (AGO- GYN8). Gynecol Oncol 2016;140:450–6.
414 Tsoref D, Welch S, Lau S, etal. Phase II study of oral ridaforolimus
in women with recurrent or metastatic endometrial cancer. Gynecol
Oncol 2014;135:184–9.
415 Matulonis U, Vergote I, Backes F, etal. Phase II study of the
PI3K inhibitor pilaralisib (SAR245408; XL147) in patients with
advanced or recurrent endometrial carcinoma. Gynecol Oncol
2015;136:246–53.
416 Aghajanian C, Filiaci V, Dizon DS, etal. A phase II study of frontline
paclitaxel/carboplatin/bevacizumab, paclitaxel/carboplatin/
temsirolimus, or ixabepilone/carboplatin/bevacizumab in advanced/
recurrent endometrial cancer. Gynecol Oncol 2018;150:274–81.
417 Sapienza LG, Ning MS, Jhingran A, etal. Short- course palliative
radiation therapy leads to excellent bleeding control: a single centre
retrospective study. Clin Transl Radiat Oncol 2019;14:40–6.
418 Klopp AH, Yeung AR, Deshmukh S, etal. Patient- reported
toxicity during pelvic intensity- modulated radiation therapy: NRG
Oncology- RTOG 1203. J Clin Oncol 2018;36:2538–44.
419 Klopp A, Smith BD, Alektiar K, etal. The role of postoperative
radiation therapy for endometrial cancer: Executive summary of
an American Society for Radiation Oncology evidence- based
guideline. Pract Radiat Oncol 2014;4:137–44.
420 Harkenrider MM, Block AM, Alektiar KM, etal. American
Brachytherapy Task Group report: adjuvant vaginal brachytherapy
for early- stage endometrial cancer: a comprehensive review.
Brachytherapy 2017;16:95–108.
421 Sturdza A, Viswanathan AN, Erickson B, etal. American
Brachytherapy Society Working Group report on the patterns of
care and a literature review of reirradiation for gynecologic cancers.
Brachytherapy 2020;19:127–38.
422 Schmid MP, Fokdal L, Westerveld H, etal. Recommendations
from gynaecological (GYN) GEC- ESTRO working group - ACROP:
target concept for image guided adaptive brachytherapy in primary
vaginal cancer. Radiother Oncol 2020;145:36–44.
423 Mendez LC, Leung E, Cheung P, etal. The role of stereotactic
ablative body radiotherapy in gynaecological cancers: a systematic
review. Clin Oncol 2017;29:378–84.
424 Hymel R, Jones GC, Simone CB. Whole pelvic intensity- modulated
radiotherapy for gynecological malignancies: a review of the
literature. Crit Rev Oncol Hematol 2015;94:371–9.
425 Köbel M, Ronnett BM, Singh N, etal. Interpretation of p53
immunohistochemistry in endometrial carcinomas: toward
increased reproducibility. Int J Gynecol Pathol 2019;38 Suppl
1:S123–31.
426 Singh N, Hirschowitz L, Zaino R, etal. Pathologic prognostic
factors in endometrial carcinoma (other than tumor type and
grade). Int J Gynecol Pathol 2019;38 Suppl 1:S93–113.
427 Malpica A, Euscher ED, Hecht JL, etal. Endometrial carcinoma,
grossing and processing issues: recommendations of the
International Society of Gynecologic Pathologists. Int J Gynecol
Pathol 2019;38 Suppl 1:S9–24.
428 McCluggage WG, Colgan T, Duggan M, etal. Data set for
reporting of endometrial carcinomas: recommendations from the
International Collaboration on Cancer Reporting (ICCR) between
United Kingdom, United States, Canada, and Australasia. Int J
Gynecol Pathol 2013;32:45–65.
429 Soslow RA, Tornos C, Park KJ, etal. Endometrial carcinoma
diagnosis: use of FIGO grading and genomic subcategories in
clinical practice: recommendations of the International Society of
Gynecological Pathologists. Int J Gynecol Pathol 2019;38 Suppl
1:S64–74.
430 Weis J, Hasenburg A. Psychological support. In: Ayhan AR,
Gultekin N, Dursun P, eds. Textbook of gynaecological oncology.
Copenhagen: Gunes Publishing, 2016: 1495–9.
431 Andersen BL, DeRubeis RJ, Berman BS, etal. Screening,
assessment, and care of anxiety and depressive symptoms in
adults with cancer: an American Society of Clinical Oncology
guideline adaptation. J Clin Oncol 2014;32:1605–19.
432 Donovan KA, Grassi L, McGinty HL, etal. Validation of the distress
thermometer worldwide: state of the science. Psychooncology
2014;23:241–50.
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2020-002230 on 18 December 2020. Downloaded from
