1455
KurnitKC, FrumovitzM. Int J Gynecol Cancer 2022;32:1455–1462. doi:10.1136/ijgc-2022-003806
Primary mucinous ovarian cancer: options for
surgery andchemotherapy
Katherine C Kurnit ,
1
Michael Frumovitz
2
1
Department of Obstetrics
and Gynecology, University of
Chicago Medicine, Chicago,
Illinois, USA
2
Department of Gynecologic
Oncology and Reproductive
Medicine, University of Texas
MD Anderson Cancer Center,
Houston, Texas, USA
Correspondence to
Dr Katherine C Kurnit,
Department of Obstetrics
and Gynecology, University of
Chicago Biological Sciences
Division, Chicago, IL 60637,
USA; kkurnit@ uchicago. edu
Received 11 July 2022
Accepted 27 September 2022
Published Online First
13October2022
To cite: KurnitKC,
FrumovitzM. Int J Gynecol
Cancer 2022;32:1455–1462.
Review
© IGCS and ESGO 2022. No
commercial re- use. See rights
and permissions. Published by
BMJ.
Original research
Editorials
Joint statement
Society statement
Meeting summary
Review articles
Consensus statement
Clinical trial
Tumor board
Video articles
Educational video lecture
Images
Pathology archives
Corners of the world
Commentary
Letters
ijgc.bmj.com
INTERNATIONAL JOURNAL OF
GYNECOLOGICAL CANCER
ABSTRACT
Primary mucinous ovarian cancer is a rare type of
epithelial ovarian cancer. In this comprehensive review we
discuss management recommendations for the treatment
of mucinous ovarian cancer. Although most tumors are
stage I at diagnosis, 15–20% are advanced stage at
diagnosis. Traditionally, patients with primary mucinous
ovarian cancer have been treated similarly to those with
the more common serous ovarian cancer. However, recent
studies have shown that mucinous ovarian cancer is very
different from other types of epithelial ovarian cancer.
Primary mucinous ovarian cancer is less likely to spread
to lymph nodes or the upper abdomen and more likely to
affect younger women, who may desire fertility- sparing
therapies. Surgical management of mucinous ovarian
cancer mirrors surgical management of other types of
epithelial ovarian cancer and includes a bilateral salpingo-
oophorectomy and total hysterectomy. When staging is
indicated, it should include pelvic washing, omentectomy,
and peritoneal biopsies; lymph node evaluation should
be considered in patients with inltrative tumors. The
appendix should be routinely evaluated intra- operatively,
but an appendectomy may be omitted if the appendix
appears grossly normal. Fertility preservation can be
considered in patients with gross disease conned to one
ovary and a normal- appearing contralateral ovary. Patients
with recurrent platinum- sensitive disease whose disease
distribution suggests a high likelihood of complete gross
resection may be candidates for secondary debulking.
Primary mucinous ovarian cancer seems to be resistant to
standard platinum- and- taxane regimens used frequently
for other types of ovarian cancer. Gastrointestinal cancer
regimens are another option; these include 5- uorouracil
and oxaliplatin, or capecitabine and oxaliplatin. Data
on heated intra- peritoneal chemotherapy (HIPEC) for
mucinous ovarian cancer are scarce, but HIPEC may
be worth considering. For patients with recurrence or
progression on rst- line chemotherapy, we advocate
enrollment in a clinical trial if one is available. For this
reason, it may be benecial to perform molecular testing in
all patients with recurrent or progressive mucinous ovarian
cancer.
BACKGROUND
Primary mucinous ovarian cancer is a rare malignancy
accounting for <3% of all epithelial ovarian cancers.
1
Because of its rarity, primary mucinous ovarian cancer
has historically been under- represented in clinical
trials for epithelial ovarian cancer, meaning that the
evidence base for treatment for primary mucinous
ovarian cancer is not strong. What has become clear
is that mucinous ovarian cancer is distinct from the
more common epithelial ovarian cancer types, such
as serous ovarian cancer, in terms of clinical behavior
and response to chemotherapy. For example, 83%
of patients with primary mucinous ovarian cancer
have ovary- conned disease at diagnosis compared
with only 4% of patients with serous ovarian cancer.
2
Patients with stage I primary mucinous ovarian cancer
have a 5- year overall survival rate approaching 90%.
3
In contrast, patients with stage II–IV disease have a
much worse prognosis than women with metastatic
serous ovarian cancer at similar stages treated with
similar chemotherapy regimens.
4 5
We review surgical and chemotherapy options for
women with primary mucinous ovarian cancer and
offer recommendations for treating women with this
disease.
EVALUATION AT DIAGNOSIS
Given the rarity of this tumor type, patients with a
new diagnosis of mucinous ovarian cancer should
have a thorough evaluation to rule out a gastroin-
testinal primary tumor. In two separate Gynecologic
Oncology Group studies, the majority of cases that
were initially thought to be mucinous ovarian carci-
noma were reclassied as gastrointestinal primary
tumors on re- review of the pathologic specimens
(55% in Gore et al and 57–63% in Zaino et al).
6 7
Although data regarding site of origin are not avail-
able from this study, another analysis of the published
literature suggested that colorectal primaries are the
most common gastrointestinal source, followed by
gastric, appendiceal, and pancreatic.
8
Thus, review of
the specimens by a gynecologic pathologist is critical.
Colonoscopy and upper gastrointestinal endoscopy
should also be performed to rule out a gastrointestinal
primary tumor.
Computed tomography (CT) of the chest, abdomen,
and pelvis should be performed for baseline staging,
as for other types of ovarian cancer. The tumor
markers CA125, carcinoembryonic antigen (CEA), and
CA19- 9 may all be useful in the diagnosis and surveil-
lance of mucinous ovarian tumors
9–13
and should be
evaluated at baseline. A ratio of CA125 level to CEA
level of >25 to 1 may be indicative of a gynecologic
primary tumor, although the positive predictive value
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2022-003806 on 13 October 2022. Downloaded from

1456
KurnitKC, FrumovitzM. Int J Gynecol Cancer 2022;32:1455–1462. doi:10.1136/ijgc-2022-003806
Review
is only 82%.
14
Unfortunately, mucinous ovarian cancer often is not
diagnosed until after surgery, at which time levels of tumor markers
may be normal even if they were elevated at baseline.
PATHOLOGIC FEATURES
The 2014 World Health Organization classication system sepa-
rates primary mucinous ovarian cancer into two sub- types: expan-
sile (conuent) and inltrative. Although available data are limited,
a recent review of the literature suggests that 50–60% of reported
mucinous ovarian tumors may exhibit inltrative histology.
15
Expan-
sile tumors exhibit conuent glandular growth with little or no inter-
vening stroma and no stromal invasion. These tumors have low
metastatic potential and are limited to the ovary in 95% of cases.
Furthermore, among patients with the expansile sub- type, <5% of
patients with stage I disease have recurrence. In contrast, inltrative
tumors have destructive stromal invasion with haphazard glands
and associated desmoplastic stromal reaction. These tumors are
more aggressive, and although 75% are stage I at diagnosis, in
15–30% of women with stage I disease they will recur.
15
Both
expansile and inltrative mucinous ovarian cancers usually stain
diffusely positive for CK7. They may also stain positive for CK20,
PAX- 8, and/or estrogen receptor, but when they stain positive, the
pattern of staining is focal or patchy, not diffuse. This contrasts with
the pattern observed in metastatic colorectal carcinoma, which
typically stains diffusely positive for CK20 and negative for CK7.
16
Tumor size and laterality may be helpful in differentiating primary
mucinous ovarian cancer from metastatic disease from a gastroin-
testinal tumor. If the tumor is unilateral and >10 cm in diameter, the
ovary is the primary tumor site in >80% of cases. If the tumor is
bilateral and/or <10 cm in diameter, the primary tumor site is in the
gastrointestinal tract in >90% of cases.
2
SURGICAL TREATMENT
Primary Treatment
Surgical management of primary mucinous ovarian cancer largely
mirrors surgical management of other types of epithelial ovarian
cancer. This typically includes a total hysterectomy, bilateral
salpingo- oophorectomy, omentectomy, and removal of any visible
tumor metastases with the goal of complete gross resection of
disease. When possible, surgery for mucinous ovarian cancer should
be performed by gynecologic oncologists. Traditionally, staging and
debulking procedures were done via laparotomy. More recently, use
of minimally invasive approaches has increased, most commonly
in patients with an isolated pelvic mass. When minimally invasive
surgery is used, care should be taken to avoid intra- abdominal
rupture and spillage, which increases the nal disease stage.
Staging Procedures
Even in patients with disease apparently conned to the pelvis,
occult peritoneal disease has been described in mucinous ovarian
carcinomas.
17
At a minimum, staging should include pelvic wash-
ings, omentectomy, and peritoneal biopsies. The role of lymphad-
enectomy is less certain in mucinous ovarian carcinoma than in
high- grade serous ovarian carcinoma. Historically, complete pelvic
and para- aortic lymphadenectomies were performed. In the 2010s,
data were published suggesting that the frequency of lymph node
metastasis in mucinous ovarian cancer is very low (0–2%),
18–21
and
thus lymphadenectomy in patients with grossly normal appearing
lymph nodes was frequently omitted. However, new data emerged
showing that, although lymph node metastases are rare in expansile
mucinous ovarian cancer, lymph node metastases may be present
in up to 30% of patients with the inltrative sub- type.
17 22
Thus, in
general, lymph node evaluation should be considered in patients
with inltrative tumors. From a practical standpoint, however, it
is difcult to determine the sub- type of mucinous ovarian cancer
intra- operatively. Frozen section analysis of mucinous ovarian
tumors is notoriously difcult, and one study indicated that the nal
diagnosis (benign vs borderline vs invasive mucinous carcinoma)
might differ from the diagnosis rendered on the basis of frozen
section evaluation in 10% of cases.
23
More realistically, knowledge
of the sub- type of mucinous ovarian cancer—inltrative or expan-
sile—may be more useful when the patient has already undergone
unilateral oophorectomy and the decision is being made whether to
re- operate for staging purposes.
Further research is needed into strategies for improving intra-
operative diagnosis and decision- making, and a staged procedure
may ultimately be required if a diagnosis cannot be conrmed
intra- operatively during the initial surgical procedure.
Appendectomy
Another intra- operative consideration is whether or not appendec-
tomy should be routinely performed. Previously, routine appen-
dectomy was recommended for any patient with a borderline or
invasive mucinous ovarian cancer to ensure that the appendix was
not the true primary tumor site. Most recent data suggest, however,
that the likelihood of an occult appendiceal primary tumor in a
patient with a normal- appearing appendix is of the order of 1% or
less.
17 24–26
Thus, we recommend that the appendix be routinely
evaluated intra- operatively, but an appendectomy may be omitted if
the appendix appears grossly normal, particularly if no gross meta-
static disease is identied.
Fertility Preservation
In primary mucinous ovarian cancer, as in other types of epithe-
lial ovarian cancer, fertility preservation with unilateral salpingo-
oophorectomy can be considered in patients with disease conned
to one ovary, who have a normal- appearing contralateral ovary, and
who desire future fertility. Given that the median age at diagnosis
is lower for patients with mucinous ovarian cancer than for those
with other types of epithelial ovarian cancer,
27 28
and given the
good prognosis for many patients with stage I mucinous ovarian
cancer,
3
desire for fertility preservation among these patients is not
uncommon. Data regarding the safety of fertility preservation are
limited, but overall small series have not shown a clear increased
risk of recurrence or death with fertility- sparing approaches.
28–31
In
these small series, some but not all patients had surgical staging
with biopsies, omentectomy, and/or lymphadenectomy, which also
makes these data more difcult to interpret. Median age at diag-
nosis for patients included in these studies was largely in the late
20s. Given the limitations of available data, patients should be care-
fully counseled about the theoretical increased risk of recurrence,
28
particularly in the case of inltrative disease.
31
However, overall for
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2022-003806 on 13 October 2022. Downloaded from

1457
KurnitKC, FrumovitzM. Int J Gynecol Cancer 2022;32:1455–1462. doi:10.1136/ijgc-2022-003806
Review
surgically staged patients desiring fertility preservation, fertility-
sparing surgeries could be considered.
Treatment of Recurrent Disease
Data regarding surgical interventions for recurrent mucinous ovarian
carcinoma are largely extrapolated from other types of epithelial
ovarian cancer. Several recent trials suggested that secondary
debulking may be benecial for patients in whom the likelihood of
achieving a complete gross resection is high,
32 33
although notably
one study that included bevacizumab did not show any survival
benet for secondary debulking.
34
It is worth noting, however, that
very few patients with mucinous ovarian cancer were included in
these studies. In general, though, patients with platinum- sensitive
disease whose disease distribution suggests a high likelihood
of complete gross resection may be candidates for secondary
debulking. Furthermore, given the poor rates of response to systemic
treatment (discussed below), we believe secondary cytoreduction
may prove to be more benecial in patients with mucinous ovarian
cancer than in patients with other types of epithelial ovarian cancer.
SYSTEMIC TREATMENT
Primary Treatment
Historically, patients with mucinous ovarian cancer were included
in large practice- changing trials of systemic treatment for other
types of epithelial ovarian cancer. For this reason, until recently,
standard rst- line adjuvant systemic treatment for patients with
mucinous ovarian cancer included the doublet of carboplatin and
paclitaxel, with consideration of adding bevacizumab for patients
with advanced disease. However, given the rarity of this histologic
type, patients with mucinous ovarian cancer frequently accounted
for only 7% or fewer of the patients in these large trials.
12 35–39
Additionally, mucinous ovarian cancer behaves markedly differ-
ently from high- grade serous ovarian cancer,
7
the type that is most
widely represented in these studies. Thus, it is uncertain whether
results from these landmark trials can truly be extrapolated to
patients with mucinous ovarian cancer.
The poorer prognosis seen with mucinous ovarian cancer relative
to high- grade serous ovarian cancer is thought to be due at least in
part to the lower degree of platinum sensitivity of mucinous ovarian
cancer. Platinum sensitivity data are largely based on retrospec-
tive studies. One such study, a case–control study that included
27 patients with advanced mucinous ovarian cancer, showed a
response rate of 26% for these patients compared with 65% in
patients with other types of epithelial ovarian cancer (the control
group).
4
A second retrospective study found that the response rate
to rst- line platinum- based chemotherapy was 39% for patients
with advanced- stage mucinous ovarian cancer compared with 70%
for patients with serous ovarian cancer.
40
In a third small study of
21 patients with newly diagnosed mucinous ovarian cancer, only
32% of evaluable patients had a complete response to rst- line
platinum- based chemotherapy and an additional 11% had a partial
response; 47% had disease progression.
10
Given these poor response rates and the histologic similarity
between primary mucinous ovarian tumors and mucinous tumors
of the gastrointestinal tract, the hypothesis arose that mucinous
ovarian tumors may respond better to gastrointestinal chemo-
therapy regimens. On the basis of pre- clinical data supporting this
hypothesis,
41
a phase II trial comparing a gastrointestinal cancer
regimen with a traditional gynecologic cancer regimen in patients
with mucinous ovarian cancer was implemented. Gynecologic
Oncology Group trial 241/mEOC randomized patients to one of
four arms: capecitabine and oxaliplatin; capecitabine, oxaliplatin,
and bevacizumab; carboplatin and paclitaxel; or carboplatin, pacl-
itaxel, and bevacizumab.
6
The planned accrual was 330 patients,
and enrollment occurred in both the USA and the UK. Unfortunately,
the study closed early, in large part because of poor accrual. This
highlights one of the major barriers to clinical trials of rare tumor
types: accrual is very difcult and frequently requires many years of
enrollment even when the trial is open nationwide or internationally.
On analyses of the enrolled patients, the investigators also iden-
tied a second signicant issue: 55% of the patients were found
to have a non- gynecologic primary mucinous tumor on central
pathology review.
6
This nding limited the conclusions that could
be drawn. However, the data that were available suggested that
gastrointestinal cancer regimens were not worse than gynecologic
cancer regimens, even when the analysis was limited to the sub-
set of patients in whom an ovarian primary tumor was conrmed.
Following these results, the results from two other retrospective
studies were published. The rst was a descriptive study of 21
patients who received adjuvant chemotherapy with either a gastro-
intestinal cancer regimen (n=9) or a gynecologic cancer regimen
(n=12).
42
No difference was seen between the two regimens but,
of note, the stage distributions for the two regimens were unbal-
anced with those receiving the gastrointestinal cancer regimen
having more advanced disease. The second study, a retrospective
cohort study from two tertiary referral centers, evaluated the use of
gynecologic cancer (n=26) and gastrointestinal cancer regimens
(n=26) as rst- line treatment for patients with mucinous ovarian
cancer. The results of this second study demonstrated a signicant
improvement in overall survival for patients who received gastro-
intestinal cancer regimens, primarily 5- uorouracil and oxaliplatin
or capecitabine and oxaliplatin, with or without bevacizumab.
43
On
the basis of these limited data, as well as the overall biological
rationale and the poor outcomes seen for patients with advanced
mucinous ovarian cancer, the National Comprehensive Cancer
Network (NCCN) has updated their recommendations for rst- line
treatment for mucinous ovarian cancer to include 5- uorouracil
and oxaliplatin, capecitabine and oxaliplatin, and carboplatin and
paclitaxel.
44
Recommendations for which patients with mucinous ovarian
cancer would benet most from adjuvant treatment are varied.
Most organizations agree that patients with stage II, III, or IV disease
should receive adjuvant treatment. In general, outcomes for this
group of patients remain poor.
28
The use of adjuvant treatment was
supported by a recent database study of patients with stages II–IV
mucinous ovarian cancer that showed an improvement in overall
survival for those who received chemotherapy.
45
However, recom-
mendations for patients with stage I disease are mixed. NCCN
recommends that patients with stage IC disease receive adjuvant
chemotherapy and that those with stage IA or IB disease do not,
regardless of other histologic ndings.
44
In contrast, the European
Society for Medical Oncology (ESMO) guidelines recommend that
treatment decisions for stage I disease be based at least in part
on the histologic sub- type, as inltrative tumors tend to behave
more aggressively than expansile tumors.
46
The ESMO guidelines
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2022-003806 on 13 October 2022. Downloaded from
1458
KurnitKC, FrumovitzM. Int J Gynecol Cancer 2022;32:1455–1462. doi:10.1136/ijgc-2022-003806
Review
recommend against adjuvant chemotherapy for patients with stage
IA expansile, grade 1–2 tumors. For all other sub- groups of patients
with stage I disease, the ESMO guidelines either state that chemo-
therapy should be considered (for patients with stage IA inltrative
tumors or stage IB or IC expansile tumors) or state that chemo-
therapy is recommended (for patients with stage IB or IC inltrative
tumors). A recent study from the National Cancer Database found
no improvement in overall survival for patients with stage I disease
who received adjuvant chemotherapy.
3
However, in that report,
the authors provided no information about histologic sub- groups.
A different study from the National Cancer Database attempted
to further characterize patients with stage I disease into higher-
and lower- risk groups, but notably also did not include information
about inltrative or expansile histology.
47
The differences in recom-
mendations regarding adjuvant chemotherapy for primary muci-
nous ovarian cancer reect the limited data that are available and
highlight an area where further investigation would be benecial.
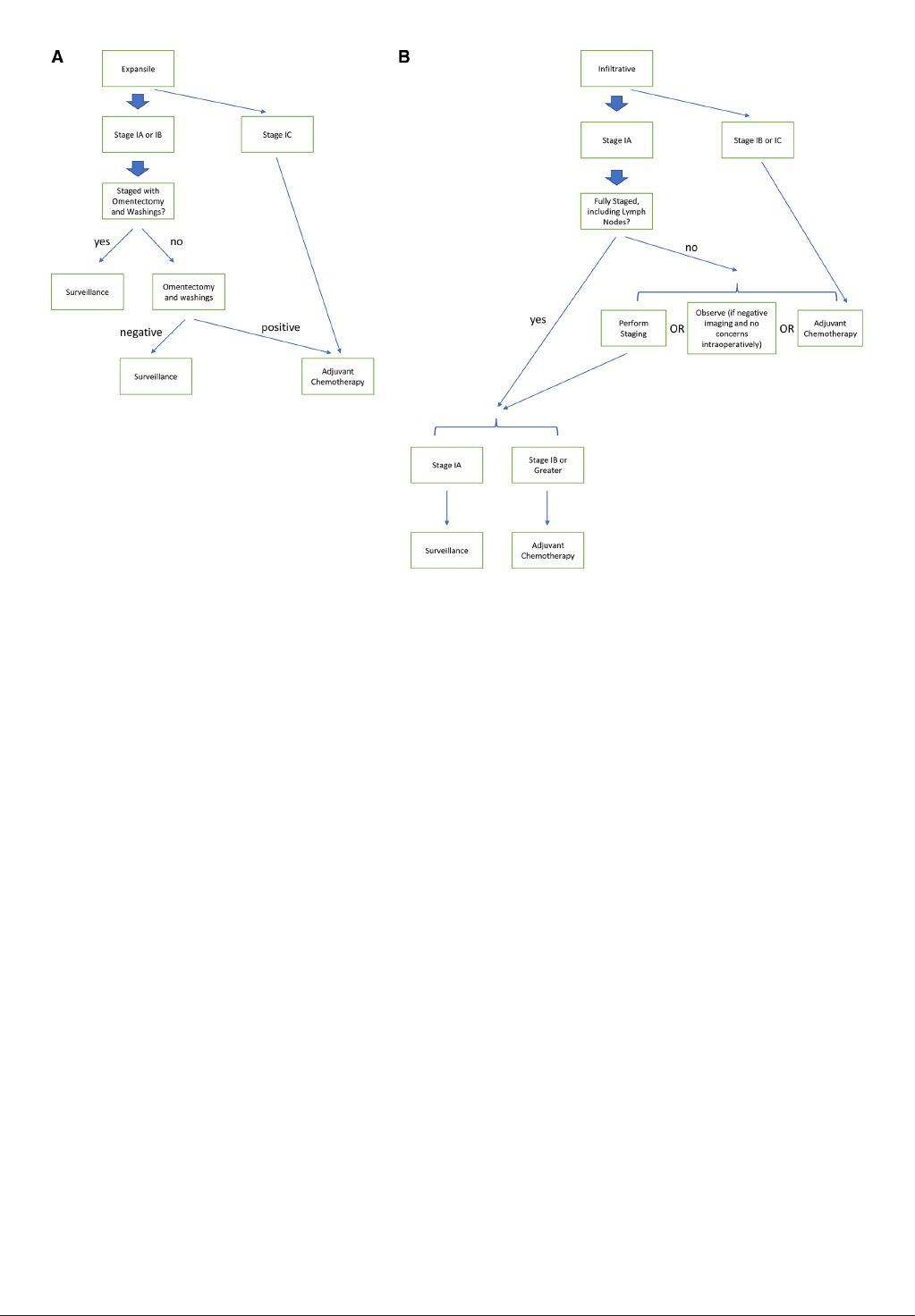
Incorporating these ndings, we would recommend erring on the
side of complete staging for patients with clinical stage I mucinous
ovarian cancer with inltrative histology. Our proposed approach is
outlined in Figure1.
Neoadjuvant chemotherapy might be appropriate for certain
patients with advanced stage mucinous ovarian cancer, with
patient selection for treatment based on algorithms for other
types of ovarian cancer
44
; however, neoadjuvant chemotherapy for
mucinous ovarian cancer remains understudied. Within the three
largest trials of neoadjuvant chemotherapy for ovarian cancer, no
more than 3% of patients had a diagnosis of mucinous ovarian
cancer.
48–50
Therefore, the safety and efcacy of neoadjuvant
chemotherapy with interval debulking surgeries in patients with
mucinous ovarian cancer remain unknown. Furthermore, it is
uncertain whether traditional gynecologic cancer regimens should
be used, or whether these patients would benet from gastrointes-
tinal cancer regimens, as discussed above. A nal consideration is
that diagnosing the primary tumor site for mucinous tumors may
be more difcult with a core biopsy and limited tissue than when
the entire ovary can be examined. Thus, in cases of metastatic
mucinous disease in which the primary tumor site of origin is not
clear, we recommend review in collaboration with a gastrointes-
tinal oncology team. However, outcomes for metastatic mucinous
carcinoma, regardless of whether it arises from a gynecologic or
gastrointestinal primary tumor, are overall poor.
7
Heated Intraperitoneal Chemotherapy (HIPEC)
Whether or not heated intraperitoneal chemotherapy (HIPEC) may
be benecial for patients with epithelial ovarian cancer has been
increasingly discussed. A study by van Driel et al in the Netherlands
showed a recurrence- free and overall survival benet from HIPEC
at the time of interval debulking for patients with stage III epithelial
ovarian cancer.
51
In contrast, a phase II trial at the Memorial Sloan
Kettering Cancer Center in the USA, which evaluated the use of
HIPEC for recurrent platinum- sensitive high- grade serous ovarian
cancer, did not nd a benet.
52
The utility of HIPEC for recurrent
high- grade serous ovarian cancer therefore remains uncertain.
Data on the use of HIPEC in patients with mucinous ovarian cancer
are currently limited. Notably, the study by van Driel et al cited
above included only three patients with mucinous ovarian cancer
among a total of 245 patients enrolled.
51
Despite these limited data,
there remains substantial interest in the use of HIPEC in patients
with mucinous ovarian cancer because of the disease’s similarity to
gastrointestinal tumors, in which HIPEC is often used to treat carci-
nomatosis.
53
The Peritoneal Surface Oncology Group International
Figure 1 Treatment algorithm for stage I mucinous ovarian cancer tumors with (A)expansile or (B)inltrative sub- types.
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2022-003806 on 13 October 2022. Downloaded from

1459
KurnitKC, FrumovitzM. Int J Gynecol Cancer 2022;32:1455–1462. doi:10.1136/ijgc-2022-003806
Review
and BIG- RENAPE working groups in France published their expe-
rience with the use of HIPEC in rare ovarian tumors. They found a
particular benet in patients with mucinous ovarian cancer; in this
group, neither the median progression- free survival nor the median
overall survival had been reached by 5 years (n=77).
54
Patients with
mucinous ovarian cancer in this study had perfusion with either
mitomycin C or oxaliplatin, which is the working groups’ standard
treatment for appendiceal tumors.
55
In contrast, all patients in the
study by van Driel et al had perfusion with cisplatin.
51
A group from China published their experience with patients
diagnosed with pseudomyxoma peritonei secondary to mucinous
ovarian cancer.
56
Over a 10- year period they performed cytoreduc-
tion and HIPEC in 22 patients. Similar to van Driel et al, this group
used cisplatin for perfusion. Patients with low- grade mucinous
cancers and those with lower peritoneal cancer index scores had
improved overall survival. Notably, median overall survival times
were shorter than 40 months for the entire cohort of patients with
pseudomyxoma peritonei due to mucinous ovarian cancer in this
study.
56
Much remains unanswered about the role of HIPEC in mucinous
ovarian cancer. Additionally, recent data about the use of HIPEC in
colorectal cancer did not show the benets anticipated,
57
implying
that there is still much to be understood about HIPEC in general.
However, given the rarity of mucinous ovarian cancer and the
lack of effective systemic treatment options, HIPEC may be worth
considering. Whether HIPEC is best given at the time of initial diag-
nosis, as a second staging surgery following the initial diagnosis in
the up- front setting, or at the time of secondary debulking is not
clear. Given that that vast majority of mucinous ovarian cancers
are stage I at diagnosis and do not recur, it is likely that primary
treatment with HIPEC for all patients with newly diagnosed muci-
nous ovarian cancer would result in overtreatment in a substantial
proportion of patients. Thus, it may make most sense to preserve
HIPEC for tumors associated with highest risk: those that are meta-
static at diagnosis or those that have already recurred. We hope
that future trials will better delineate the optimal use of HIPEC in
patients with mucinous ovarian cancer.
Treatment of Recurrent and Progressive Disease
Data on second- line systemic treatment for mucinous ovarian
cancer are even more limited than data on rst- line systemic treat-
ment. A retrospective study evaluated 20 patients with mucinous
ovarian cancer who had a recurrence at least 6 months after their
initial treatment.
9
Although the number of patients in this study
was small, several patients treated with platinum as second-
line chemotherapy had a response. No patient treated with non-
platinum agents as second- line chemotherapy had a response.
Among patients treated with third- or fourth- line chemotherapy,
one patient had a response to paclitaxel, topotecan, and cyclophos-
phamide as third- line treatment, and one patient had a response
to this regimen as fourth- line treatment, but no responses were
seen to gemcitabine or liposomal doxorubicin.
9
Another small study
found that none of the 12 patients treated in second line or beyond
with cytotoxic chemotherapy had a complete response, and only
one and two patients had partial and complete responses, respec-
tively, to second- line treatment. The remaining eight patients in the
second- line setting and six patients in the third- line setting all had
progression of disease.
10
Bevacizumab may also be benecial for treatment of recurrent or
progressive disease. One case report described a patient with recur-
rence after adjuvant carboplatin and paclitaxel followed by several
standard single- agent regimens for platinum- resistant disease.
She was treated with weekly paclitaxel with bevacizumab for six
cycles, followed by maintenance bevacizumab for 32 cycles, and
she had a durable response to treatment (stable disease).
58
Another
case report described durable disease stabilization of a borderline
mucinous ovarian tumor with single- agent bevacizumab.
59
In general, responses to standard- of- care chemotherapy at the
time of recurrence or progression of mucinous ovarian cancer
are relatively poor. Other regimens for platinum- resistant ovarian
cancer listed in the NCCN guidelines should be considered. For
patients who received combinations of platinum agents and
taxanes for adjuvant treatment, there may be benet to attempting
a gastrointestinal cancer regimen such as capecitabine and oxal-
iplatin or 5- uorouracil and oxaliplatin. However, given the poor
outcomes described above, even for patients who previously
received gastrointestinal cancer regimens, it may also be benecial
to extrapolate from data on mucinous gastrointestinal tumors and
consider second- line gastrointestinal cancer regimens, particularly
in patients whose functional status remains good after receipt of
multiple other lines of treatment. Further data are needed to explore
the question of whether the sequence of regimens matters and
which second- line regimens are most likely to be successful.
Molecular Characterization and Novel Agents
Given the poor response of mucinous ovarian cancer to cytotoxic
chemotherapy, there is much interest in further exploring novel
agents for this rare tumor type. Large molecular studies have
shown that mucinous ovarian cancers often have aberrations in
TP53 (57–90%), KRAS (44–79%), and CDKN2A (11–19%).
60–64
Her2 positivity or ERBB2 amplication has also been demonstrated
in 18–27% of mucinous ovarian cancers.
62 63 65 66
Mismatch repair deciency has been reported in mucinous
ovarian cancer, although it is a rare event.
60 67
Thus, the tumor-
type agnostic approval of pembrolizumab for patients with tumors
demonstrating mismatch repair deciency or high microsatel-
lite instability
68 69
provides another viable option for treatment of
recurrent disease. Homologous recombination deciency, which is
present in up to 50% of high- grade serous ovarian cancers,
70
is
rare in mucinous ovarian tumors.
67
Her2 positivity may be targetable given its prevalence described
above, although data about treatment options for Her2- positive
disease are limited. Extrapolating from other tumor types, it may
be possible to combine Her2- targeting treatment with standard- of-
care chemotherapy (trastuzumab plus carboplatin and paclitaxel
71
)
or to give Her2- targeting treatment as a single agent. Additionally,
the combination of trastuzumab and pertuzumab is currently being
explored in other tumor types with Her2 positivity,
72 73
and while this
combination has not been evaluated in mucinous ovarian cancer, it
may represent an avenue for future exploration.
Estrogen and progesterone receptor positivity has also been
reported in a sub- set of patients with mucinous ovarian cancer
in one study,
67 74
and thus anti- hormonal agents could be consid-
ered for selected patients. Novel agents targeting the Ras pathway
are currently in use for other tumor types and may be a prom-
ising avenue in the future. Epidermal growth factor receptor (EGFR)
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2022-003806 on 13 October 2022. Downloaded from

1460
KurnitKC, FrumovitzM. Int J Gynecol Cancer 2022;32:1455–1462. doi:10.1136/ijgc-2022-003806
Review
inhibitors have also been proposed,
75
and other new treatments are
being explored in pre- clinical studies.
76–80
While data are lacking
for many of these novel agents, we hope that patients with muci-
nous ovarian cancers will be enrolled in future basket trials of
these targeted therapies as it is unlikely that a biomarker- driven,
tumor type- specic trial of treatment for this rare histologic sub-
type could achieve adequate accrual. If a patient has recurrence or
progression on rst- line chemotherapy, we would advocate enroll-
ment in a clinical trial if one is available. For this reason, it may be
benecial to perform molecular testing, including next- generation
sequencing, mismatch repair deciency, and Her2 testing, in all
patients with recurrent or progressive mucinous ovarian cancer.
CONCLUSIONS
Metastatic mucinous ovarian cancer is a rare but aggressive type
of epithelial ovarian cancer. Given its rarity, large prospective
studies of treatment for this disease have been difcult. This review
summarizes the available data and highlights areas that warrant
future investigation. Many patients are diagnosed at an early stage
and outcomes in this setting are better; however, identifying the
ideal algorithms for patients with early- stage inltrative disease
has been difcult. Similarly, surgical management may need to
differ depending on histologic ndings, so developing ways that
details about the histologic sub- type can be available at the time of
a surgical staging procedure could be important. Although systemic
treatment is recommended for all patients with metastatic muci-
nous ovarian cancer, data are still limited on whether a traditional
gynecologic regimen is sufcient or whether a gastrointestinal
regimen may be benecial. Fully dening the role of HIPEC and
knowledge about which second- line regimens are most effective
have also remained elusive. New therapies and updated treat-
ment algorithms are sorely needed, and we may need to rely on
novel clinical trial designs and basket trial approaches in order to
obtain the data we need to move the eld forward for this sub- set
of patients.
Twitter Michael Frumovitz @frumovitz
Contributors KK: concept development, literature search, writing, revisions,
guarantor. MF: concept development, literature search, writing, revisions.
Funding The authors have not declared a specic grant for this research from any
funding agency in the public, commercial or not- for- prot sectors.
Competing interests None declared.
Patient consent for publication Not applicable.
Ethics approval Not applicable.
Provenance and peer review Not commissioned; externally peer reviewed.
ORCID iDs
Katherine CKurnit http://orcid.org/0000-0003-3205-0128
MichaelFrumovitz http://orcid.org/0000-0002-0810-2648
REFERENCES
1 Seidman JD, Horkayne- Szakaly I, Haiba M, etal. The histologic type
and stage distribution of ovarian carcinomas of surface epithelial
origin. Int J Gynecol Pathol 2004;23:41–4.
2 Seidman JD, Kurman RJ, Ronnett BM. Primary and metastatic
mucinous adenocarcinomas in the ovaries: incidence in routine
practice with a new approach to improve intraoperative diagnosis.
Am J Surg Pathol 2003;27:985–93.
3 Nasioudis D, Haggerty AF, Giuntoli RL, etal. Adjuvant chemotherapy
is not associated with a survival benet for patients with early stage
mucinous ovarian carcinoma. Gynecol Oncol 2019;154:302–7.
4 Hess V, A'Hern R, Nasiri N, etal. Mucinous epithelial ovarian
cancer: a separate entity requiring specic treatment. J Clin Oncol
2004;22:1040–4.
5 Winter WE, Maxwell GL, Tian C, etal. Prognostic factors for stage III
epithelial ovarian cancer: a Gynecologic Oncology Group study.
J Clin Oncol 2007;25:3621–7.
6 Gore M, Hackshaw A, Brady WE, etal. An international, phase III
randomized trial in patients with mucinous epithelial ovarian cancer
(mEOC/GOG 0241) with long- term follow- up: and experience of
conducting a clinical trial in a rare gynecological tumor. Gynecol
Oncol 2019;153:541–8.
7 Zaino RJ, Brady MF, Lele SM, etal. Advanced stage mucinous
adenocarcinoma of the ovary is both rare and highly lethal: a
Gynecologic Oncology Group study. Cancer 2011;117:554–62.
8 Dundr P, Singh N, Nožičková B, etal. Primary mucinous ovarian
tumors vs. ovarian metastases from gastrointestinal tract, pancreas
and biliary tree: a review of current problematics. Diagn Pathol
2021;16:20.
9 Pignata S, Ferrandina G, Scarfone G, etal. Activity of chemotherapy
in mucinous ovarian cancer with a recurrence free interval of more
than 6 months: results from the SOCRATES retrospective study.
BMC Cancer 2008;8:252.
10 Pisano C, Greggi S, Tambaro R, etal. Activity of chemotherapy in
mucinous epithelial ovarian cancer: a retrospective study. Anticancer
Res 2005;25:3501–5.
11 McCluggage WG. Immunohistochemistry in the distinction between
primary and metastatic ovarian mucinous neoplasms. J Clin Pathol
2012;65:596–600.
12 Xu W, Rush J, Rickett K, etal. Mucinous ovarian cancer: a
therapeutic review. Crit Rev Oncol Hematol 2016;102:26–36.
13 Kelly PJ, Archbold P, Price JH, etal. Serum CA19.9 levels are
commonly elevated in primary ovarian mucinous tumours but
cannot be used to predict the histological subtype. J Clin Pathol
2010;63:169–73.
14 Sørensen SS, Mosgaard BJ. Combination of cancer antigen 125 and
carcinoembryonic antigen can improve ovarian cancer diagnosis.
Dan Med Bull 2011;58:A4331.
15 Morice P, Gouy S, Leary A. Mucinous ovarian carcinoma. N Engl J
Med 2019;380:1256–66.
16 Babaier A, Ghatage P. Mucinous cancer of the ovary: overview
and current status. Diagnostics 2020;10. doi:10.3390/
diagnostics10010052. [Epub ahead of print: 19 01 2020].
17 Gouy S, Saidani M, Maulard A, etal. Staging surgery in early- stage
ovarian mucinous tumors according to expansile and inltrative
types. Gynecol Oncol Rep 2017;22:21–5.
18 Schmeler KM, Tao X, Frumovitz M, etal. Prevalence of lymph node
metastasis in primary mucinous carcinoma of the ovary. Obstet
Gynecol 2010;116:269–73.
19 Salgado- Ceballos I, Ríos J, Pérez- Montiel D, etal. Is
lymphadenectomy necessary in mucinous ovarian cancer? A single
institution experience. Int J Surg 2017;41:1–5.
20 van Baal J, Van de Vijver KK, Coffelt SB, etal. Incidence of lymph
node metastases in clinical early- stage mucinous and seromucinous
ovarian carcinoma: a retrospective cohort study. BJOG
2017;124:486–94.
21 Hoogendam JP, Vlek CA, Witteveen PO, etal. Surgical lymph node
assessment in mucinous ovarian carcinoma staging: a systematic
review and meta- analysis. BJOG 2017;124:370–8.
22 Muyldermans K, Moerman P, Amant F, etal. Primary invasive
mucinous ovarian carcinoma of the intestinal type: importance of the
expansile versus inltrative type in predicting recurrence and lymph
node metastases. Eur J Cancer 2013;49:1600–8.
23 Park JY, Lee SH, Kim KR, etal. Accuracy of frozen section diagnosis
and factors associated with nal pathological diagnosis upgrade of
mucinous ovarian tumors. J Gynecol Oncol 2019;30:e95.
24 Lin JE, Seo S, Kushner DM, etal. The role of appendectomy for
mucinous ovarian neoplasms. Am J Obstet Gynecol 2013;208:46
e1–4.
25 Ozcan A, Töz E, Turan V, etal. Should we remove the normal-
looking appendix during operations for borderline mucinous
ovarian neoplasms? A retrospective study of 129 cases. Int J Surg
2015;18:99–103.
26 Cheng A, Li M, Kanis MJ, etal. Is it necessary to perform routine
appendectomy for mucinous ovarian neoplasms? A retrospective
study and meta- analysis. Gynecol Oncol 2017;144:215–22.
27 Peres LC, Cushing- Haugen KL, Köbel M, etal. Invasive epithelial
ovarian cancer survival by histotype and disease stage. J Natl
Cancer Inst 2019;111:60–8.
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2022-003806 on 13 October 2022. Downloaded from

1461
KurnitKC, FrumovitzM. Int J Gynecol Cancer 2022;32:1455–1462. doi:10.1136/ijgc-2022-003806
Review
28 Mueller JJ, Lajer H, Mosgaard BJ, etal. International study of
primary mucinous ovarian carcinomas managed at tertiary medical
centers. Int J Gynecol Cancer 2018;28:915–24.
29 Lee J- Y, Jo YR, Kim TH, etal. Safety of fertility- sparing surgery
in primary mucinous carcinoma of the ovary. Cancer Res Treat
2015;47:290–7.
30 Gouy S, Saidani M, Maulard A, etal. Results of fertility- sparing
surgery for expansile and inltrative mucinous ovarian cancers.
Oncologist 2018;23:324–7.
31 Gouy S, Saidani M, Maulard A, etal. Is uterine preservation
combined with bilateral salpingo- oophorectomy to promote
subsequent fertility safe in inltrative mucinous ovarian cancer?
Gynecol Oncol Rep 2017;22:52–4.
32 Shi T, Zhu J, Feng Y, etal. Secondary cytoreduction followed by
chemotherapy versus chemotherapy alone in platinum- sensitive
relapsed ovarian cancer (SOC- 1): a multicentre, open- label,
randomised, phase 3 trial. Lancet Oncol 2021;22:439–49.
33 Harter P, Sehouli J, Vergote I, etal. Randomized trial of
cytoreductive surgery for relapsed ovarian cancer. N Engl J Med
2021;385:2123–31.
34 Coleman RL, Spirtos NM, Enserro D, etal. Secondary surgical
cytoreduction for recurrent ovarian cancer. N Engl J Med
2019;381:1929–39.
35 Perren TJ, Swart AM, Psterer J, etal. A phase 3 trial of
bevacizumab in ovarian cancer. N Engl J Med 2011;365:2484–96.
36 McGuire WP, Hoskins WJ, Brady MF, etal. Cyclophosphamide and
cisplatin compared with paclitaxel and cisplatin in patients with
stage III and stage IV ovarian cancer. N Engl J Med 1996;334:1–6.
37 Muggia FM, Braly PS, Brady MF, etal. Phase III randomized study of
cisplatin versus paclitaxel versus cisplatin and paclitaxel in patients
with suboptimal stage III or IV ovarian cancer: a Gynecologic
Oncology Group study. J Clin Oncol 2000;18:106–15.
38 Neijt JP, Engelholm SA, Tuxen MK, etal. Exploratory phase III study
of paclitaxel and cisplatin versus paclitaxel and carboplatin in
advanced ovarian cancer. J Clin Oncol 2000;18:3084–92.
39 International Collaborative Ovarian Neoplasm Group. Paclitaxel plus
carboplatin versus standard chemotherapy with either single- agent
carboplatin or cyclophosphamide, doxorubicin, and cisplatin in
women with ovarian cancer: the ICON3 randomised trial. Lancet
2002;360:505–15.
40 Pectasides D, Fountzilas G, Aravantinos G, etal. Advanced stage
mucinous epithelial ovarian cancer: the Hellenic Cooperative
Oncology Group experience. Gynecol Oncol 2005;97:436–41.
41 Sato S, Itamochi H, Kigawa J, etal. Combination chemotherapy
of oxaliplatin and 5- uorouracil may be an effective regimen for
mucinous adenocarcinoma of the ovary: a potential treatment
strategy. Cancer Sci 2009;100:546–51.
42 Schlappe BA, Zhou QC, O'Cearbhaill R, etal. A descriptive report
of outcomes of primary mucinous ovarian cancer patients receiving
either an adjuvant gynecologic or gastrointestinal chemotherapy
regimen. Int J Gynecol Cancer 2019. doi:10.1136/ijgc-2018-000150.
[Epub ahead of print: 15 May 2019].
43 Kurnit KC, Sinno AK, Fellman BM, etal. Effects of gastrointestinal-
type chemotherapy in women with ovarian mucinous carcinoma.
Obstet Gynecol 2019;134:1253–9.
44 National Comprehensive Cancer Network. Ovarian cancer including
fallopian tube cancer and primary peritoneal cancer (version 1.2022);
2022.
45 Nasioudis D, Albright BB, Ko EM, etal. Advanced stage primary
mucinous ovarian carcinoma. Where do we stand ? Arch Gynecol
Obstet 2020;301:1047–54.
46 Colombo N, Sessa C, du Bois A, etal. ESMO- ESGO consensus
conference recommendations on ovarian cancer: pathology and
molecular biology, early and advanced stages, borderline tumours
and recurrent disease†. Ann Oncol 2019;30:672–705.
47 Richardson MT, Mysona DP, Klein DA, etal. Long term survival
outcomes of stage I mucinous ovarian cancer - a clinical
calculator predictive of chemotherapy benet. Gynecol Oncol
2020;159:118–28.
48 Kehoe S, Hook J, Nankivell M, etal. Primary chemotherapy versus
primary surgery for newly diagnosed advanced ovarian cancer
(CHORUS): an open- label, randomised, controlled, non- inferiority
trial. Lancet 2015;386:249–57.
49 Vergote I, Tropé CG, Amant F, etal. Neoadjuvant chemotherapy
or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med
2010;363:943–53.
50 Fagotti A, Ferrandina MG, Vizzielli G, etal. Randomized trial of
primary debulking surgery versus neoadjuvant chemotherapy for
advanced epithelial ovarian cancer (SCORPION- NCT01461850). Int
J Gynecol Cancer 2020;30:1657–64.
51 van Driel WJ, Koole SN, Sikorska K, etal. Hyperthermic
intraperitoneal chemotherapy in ovarian cancer. N Engl J Med
2018;378:230–40.
52 Zivanovic O, Chi DS, Zhou Q, etal. Secondary cytoreduction and
carboplatin hyperthermic intraperitoneal chemotherapy for platinum-
sensitive recurrent ovarian cancer: an MSK team ovary phase II
study. J Clin Oncol 2021;39:2594–604.
53 Iavazzo C, Spiliotis J. Is there a promising role of HIPEC in patients
with advanced mucinous ovarian cancer? Arch Gynecol Obstet
2021;303:597–8.
54 Mercier F, Bakrin N, Bartlett DL, etal. Peritoneal carcinomatosis
of rare ovarian origin treated by cytoreductive surgery and
hyperthermic intraperitoneal chemotherapy: a multi- institutional
cohort from PSOGI and BIG- RENAPE. Ann Surg Oncol
2018;25:1668–75.
55 Chua TC, Moran BJ, Sugarbaker PH, etal. Early- and long- term
outcome data of patients with pseudomyxoma peritonei from
appendiceal origin treated by a strategy of cytoreductive surgery
and hyperthermic intraperitoneal chemotherapy. J Clin Oncol
2012;30:2449–56.
56 Fu F, Ma X, Lu Y, etal. Clinicopathological characteristics and
prognostic prediction in pseudomyxoma peritonei originating from
mucinous ovarian cancer. Front Oncol 2021;11:641053.
57 Quénet F, Elias D, Roca L, etal. Cytoreductive surgery plus
hyperthermic intraperitoneal chemotherapy versus cytoreductive
surgery alone for colorectal peritoneal metastases (PRODIGE 7): a
multicentre, randomised, open- label, phase 3 trial. Lancet Oncol
2021;22:256–66.
58 Tarumi Y, Mori T, Matsushima H, etal. Long- term survival with
bevacizumab in heavily pretreated and platinum- resistant
mucinous ovarian cancer: a case report. J Obstet Gynaecol Res
2018;44:347–51.
59 Winer I, Buckanovich RJ. A case of progressive mucinous ovarian
cancer of low malignant potential responsive to biologic therapy with
bevacizumab. Gynecol Oncol 2010;116:578–9.
60 Mueller JJ, Schlappe BA, Kumar R, etal. Massively parallel
sequencing analysis of mucinous ovarian carcinomas:
genomic profiling and differential diagnoses. Gynecol Oncol
2018;150:127–35.
61 Mackenzie R, Kommoss S, Winterhoff BJ, etal. Targeted deep
sequencing of mucinous ovarian tumors reveals multiple overlapping
RAS- pathway activating mutations in borderline and cancerous
neoplasms. BMC Cancer 2015;15:415.
62 Cheasley D, Wakeeld MJ, Ryland GL, etal. The molecular origin
and taxonomy of mucinous ovarian carcinoma. Nat Commun
2019;10:3935.
63 Anglesio MS, Kommoss S, Tolcher MC, etal. Molecular
characterization of mucinous ovarian tumours supports a stratied
treatment approach with HER2 targeting in 19% of carcinomas.
J Pathol 2013;229:111–20.
64 Ryland GL, Hunter SM, Doyle MA, etal. Mutational landscape of
mucinous ovarian carcinoma and its neoplastic precursors. Genome
Med 2015;7:87.
65 Chay W- Y, Chew S- H, Ong W- S, etal. HER2 amplication and
clinicopathological characteristics in a large Asian cohort of rare
mucinous ovarian cancer. PLoS One 2013;8:e61565.
66 McAlpine JN, Wiegand KC, Vang R, etal. HER2 overexpression and
amplication is present in a subset of ovarian mucinous carcinomas
and can be targeted with trastuzumab therapy. BMC Cancer
2009;9:433.
67 Gorringe KL, Cheasley D, Wakefield MJ, etal. Therapeutic
options for mucinous ovarian carcinoma. Gynecol Oncol
2020;156:552–60.
68 Le DT, Uram JN, Wang H, etal. PD- 1 blockade in tumors with
mismatch- repair deficiency. N Engl J Med 2015;372:2509–20.
69 Marcus L, Lemery SJ, Keegan P, etal. FDA approval summary:
pembrolizumab for the treatment of microsatellite instability- high
solid tumors. Clin Cancer Res 2019;25:3753–8.
70 Cancer Genome Atlas Research Network. Integrated genomic
analyses of ovarian carcinoma. Nature 2011;474:609–15.
71 Fader AN, Roque DM, Siegel E, etal. Randomized phase II trial of
carboplatin- paclitaxel versus carboplatin- paclitaxel- trastuzumab
in uterine serous carcinomas that overexpress human epidermal
growth factor receptor 2/neu. J Clin Oncol 2018;36:2044–51.
72 Javle M, Borad MJ, Azad NS, etal. Pertuzumab and trastuzumab
for HER2- positive, metastatic biliary tract cancer (MyPathway): a
multicentre, open- label, phase 2A, multiple basket study. Lancet
Oncol 2021;22:1290–300.
73 Swain SM, Miles D, Kim S- B, etal. Pertuzumab, trastuzumab,
and docetaxel for HER2- positive metastatic breast cancer
(CLEOPATRA): end- of- study results from a double- blind,
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2022-003806 on 13 October 2022. Downloaded from

1462
KurnitKC, FrumovitzM. Int J Gynecol Cancer 2022;32:1455–1462. doi:10.1136/ijgc-2022-003806
Review
randomised, placebo- controlled, phase 3 study. Lancet Oncol
2020;21:519–30.
74 Tkalia IG, Vorobyova LI, Svintsitsky VS, etal. Clinical signicance of
hormonal receptor status of malignant ovarian tumors. Exp Oncol
2014;36:125–33.
75 Jain A, Ryan PD, Seiden MV. Metastatic mucinous ovarian cancer
and treatment decisions based on histology and molecular
markers rather than the primary location. J Natl Compr Canc Netw
2012;10:1076–80.
76 Liu T, Hu W, Dalton HJ, etal. Targeting SRC and tubulin in mucinous
ovarian carcinoma. Clin Cancer Res 2013;19:6532–43.
77 Matsuo K, Nishimura M, Bottsford- Miller JN, etal. Targeting SRC in
mucinous ovarian carcinoma. Clin Cancer Res 2011;17:5367–78.
78 Niiro E, Morioka S, Iwai K, etal. Potential signaling pathways as
therapeutic targets for overcoming chemoresistance in mucinous
ovarian cancer. Biomed Rep 2018;8:215–23.
79 Ricci F, Guffanti F, Affatato R, etal. Establishment of patient- derived
tumor xenograft models of mucinous ovarian cancer. Am J Cancer
Res 2020;10:572–80.
80 Hisamatsu T, McGuire M, Wu SY, etal. PRKRA/PACT expression
promotes chemoresistance of mucinous ovarian cancer. Mol Cancer
Ther 2019;18:162–72.
on August 30, 2024 by guest. Protected by copyright.http://ijgc.bmj.com/Int J Gynecol Cancer: first published as 10.1136/ijgc-2022-003806 on 13 October 2022. Downloaded from
